2° Clinical case: Pineal Cavernoma
2° Clinical case: Pineal Cavernoma
Abstract:This chapter focuses on the clinical case of a 32-year-old man suffering from severe nocturnal and diurnal bruxism, coupled with chronic bilateral orofacial pain (OP), predominantly on the left side. Unlike the traditional approach that views bruxism solely as a dental issue, this case study adopts a broader neurophysiological perspective. It introduces the phenomenon of dystonia and its connection to bruxism, highlighting the overlapping effects of temporomandibular disorders (TMDs), headaches, and neurological factors such as hyperexcitability of the central nervous system (CNS).
The patient, after 15 years of bruxism treatment involving bite planes, presented with a worsening condition that included trunk and limb muscle stiffness, visual disturbances, and neurological abnormalities such as diplopia, nystagmus, and polykinetic tendon reflexes. The chapter contrasts dental and neurological findings, using a formal logical diagnostic framework to clarify the dominance of neurological over dental factors in this case.
Key diagnostic tools included electromyography (EMG), motor evoked potentials (MEPs), and jaw jerk reflex testing, which indicated trigeminal system involvement. The coherence demarcator (Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau} ), previously applied to the case of hemimasticatory spasm, was employed here to resolve conflicts between dental and neurological assertions, leading to a stronger focus on neurophysiological abnormalities.
The chapter concludes by proposing a neurophysiological path to decode the "machine language" of the CNS in bruxism, aiming to understand the condition not merely as a dental issue but as a possible manifestation of hyperreflexia and CNS hyperexcitability. The next diagnostic step will be elaborated in the following chapter, "Encrypted Code: Hyperexcitability of the Trigeminal System."
Introduction to the bruxist case report
As anticipated in the chapter 'Bruxism' we will avoid indicating this disorder as an exclusive dental correlate and will seek a broader and essentially more neurophysiological description by making a brief excursus on dystonic phenomena, on 'Orofacial Pain' and only then will we consider the phenomenon 'bruxism' true and own. Subsequently we will move on to the presentation of the clinical case.
Dystonia is an involuntary, repetitive, sustained (tonic), or spasmodic (rapid or clonic) muscle contraction. The spectrum of dystonias can involve various regions of the body. Of interest to oral and maxillofacial surgeons are the cranial-cervical dystonias, in particular, orofacial dystonia (OFD). OFD is an involuntary, sustained contraction of the periorbital, facial, oromandibular, pharyngeal, laryngeal, or cervical muscles.[1] OFD can involve the masticatory, lower facial, and tongue muscles, which may result in trismus, bruxism, involuntary jaw opening or closure, and involuntary tongue movement.
The etiology of OFD is varied and includes genetic predisposition, injury to the central nervous system (CNS), peripheral trauma, medications, metabolic or toxic states, and neurodegenerative disease. However, in the majority of patients, no specific cause can be identified. An association was found among painful temporomandibular disorders (TMDs), migraine, tension-type headache, and sleep bruxism, although the association was only significant for chronic migraine. The association between painful TMDs and sleep bruxism significantly increased the risk for chronic migraine, followed by episodic migraine and episodic tension-type headache.[2]
Bruxism is the most frequently occurring oral movement disorder, and can occur in subjects while awake and during sleep. Both forms are likely to have different etiologies, and their diagnosis and treatment require different approaches. Treatment is indicated when bruxism causes pain in the masticatory system or leads to damage such as tooth wear or fractures of teeth, restorations, or even of implants. A focused review on the etiology of bruxism[3] concluded that there is a limited role for morphological factors in the etiology of bruxism, while psychological factors (e.g., stress) and pathophysiological factors (e.g., disturbances in central neurotransmitter systems) are more prominently involved.
Orofacial pain (OP), including pain from TMDs, exerts a modulatory effect on mandibular stretch reflexes.[4] Electrophysiological studies have shown that experimentally induced pain from injections of 5% hypertonic saline solution into the masseter muscle causes an increase in the peak-to-peak amplitude of the jaw jerk. This facilitatory effect appears to be related to an increased sensitivity of the fusimotor system, which at the same time causes muscle stiffness.[5] In addition, a number of animal studies of experimentally-induced muscle pain have shown that activation of the muscle nociceptors markedly influences the proprioceptive properties of the muscle spindles through a central neural pathway,[6] and that washing of the local algogenic substance causes a return to normal tendon reflexes.
However, few studies have attempted to characterize the pain associated with bruxism (i.e., to examine the neurobiological and physiological characteristics of the mandibular muscles). Some clinical cases and small-scale studies suggest that certain drugs linked to the dopaminergic, serotoninergic, and adrenergic systems can either suppress or exacerbate bruxism. Further, the majority of these pharmacological studies indicate that various classes of drugs can influence the muscular activity related to bruxism, without exerting any effect on OP.[7]
Therefore, the sensitization of the trigeminal nociceptive system and the facilitating effect on mandibular stretch reflexes and CNS hyperexcitability are neurophysiopathogenetic phenomena that can be correlated to pain in the craniofacial region. However, up to now, no correlation has been reported between OP, dysfunction of the mesencephalic nuclei, and facilitation of trigeminal nociception, except for a clinical study on a patient affected by pontine cavernoma, which highlighted a relative facilitation of the trigeminal nociceptive system through the blink reflex.[8]
Bruxist Case report
As anticipated we will take up the same diagnostic language presented for the patient Mary Poppins so that it becomes an assimilable and practicable model, and we will try to superimpose it on the present clinical case called 'Bruxer'.
The subject was a 32-year-old man suffering from pronounced nocturnal and diurnal bruxism and chronic bilateral OP prevalent in the temporoparietal regions, with greater intensity and frequency on the left side. The patient came to our observation after being treated for 15 years by dental colleagues with a biteplane. A sort of muscular stiffening of the trunk and legs had recently been added to bruxism and orofacial pain. Come to our observation beyond the clinical signs of bruxism the patient, to neurological examination, showed a contraction of the masseter muscles with pronounced stiffness of the jaw, diplopia and loss of visual acuity in the left eye, left gaze nystagmus with a rotary component, papillae with blurred borders and positive bilateral Babynski’s, and polykinetic tendon reflexes in all four limbs.
From what has been exposed in the previous chapters from the 'Introduction' to the chapters 'Logic of medical language' and the last chapter 'Bruxism', in addition to the complexity of the arguments and the vagueness of the verbal language, we could find ourselves faced with a clinical situation in which seems to dominate one of the contexts considered.
(it looks like it but....)
Unlike the patient with 'Hemimasticatory Spasm', the clinical case of our poor 'Bruxer' shows a phenomenon of overlapping of propositions, assertions and logical sentences in the dental and neurological context and apparently neither of the two obtains an absolute and clear compatibility and coherence. This has repercussions in the clinic in which all the actors involved (medical examiners) are right and contextually wrong, making the diagnostic conclusion inadequate and dangerous, but let's see the process as a whole step by step.
Significance of contexts
Dental Contest significance
In the dental context we will have the following sentences and statements to which we give a numerical value to facilitate the treatment, namely Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_n=[0|1]} where it Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_n=0} indicates 'normal' e Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_n=1} abnormality and therefore positivity of the report:
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_1} Negative MR report of the TMJ in Figure 2, Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_1=0\longrightarrow} Normality, negativity of the report
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_2} Negative axiographic report for right condylar traces in Figure 3, Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_2=0\longrightarrow} Normality, negativity of the report
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_3} Negative axiographic report for left condylar traces in Figure 4, Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_3=0\longrightarrow} Normality, negativity of the report
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_4} Symmetric EMG interference pattern in Figure 5, Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_4 =0\longrightarrow} Normality, negativity of the report
(and it is precisely here that the contexts conflict or rather the results may not be so decisive)
Neurophysiological Contest significance
In the neurological context we will therefore have the following sentences and statements to which we give a numerical value to facilitate the treatment, i.e. Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_n=[0|1]} where Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_n=0} indicates 'normality' and Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_n=1} 'abnormality and therefore positivity of the report:
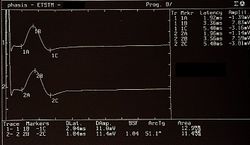
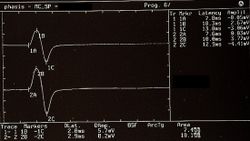
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_1=} Presence and symmetry of the Motor Evoked Potentials of the trigeminal roots in Figure 5, Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_0=0\longrightarrow} Normality, negativity of the report
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_2=} Presence of jaw jerk with relative amplitude asymmetry in Figure6 Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_2=1\longrightarrow} Abnormality, negativity of the report* (the * was inserted to note an ambiguity in the report which we will describe in detail in the clinical discussion)
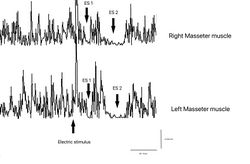
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_3=} Electrical silent period and contextual symmetry Figures 7, Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_3=0\longrightarrow} Normality, negativity of the report
Demarcator of Diagnostic Coherence Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau}
As we described in the chapter '1st Clinical case: Hemimasticatory spasm' the Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau} is a representative clinical specific weight, complex to research and develop because it varies from discipline to discipline and for pathologies, essential in order not to collide the logical assertions Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_o} and Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_n} in diagnostic procedures and fundamental to initialize the decryption of the machine language code. Basically it allows you to confirm the coherence of a union Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im\cup\{\delta_1,\delta_2.....\delta_n\}} versus another Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im\cup\{\gamma_1,\gamma_2.....\gamma_n\}} and vice versa, giving greater weight to the seriousness of the allegations and the report in the appropriate context.
The demarcation weight Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau} , therefore, gives more significance to the more serious assertions in the clinical context from which they derive and therefore beyond the greater or lesser positivity of the assertions Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 0\leq\delta_n\leq1} or Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 0\leq\gamma_n\leq1} which in any case are always verified and respected, these must be validated according to the intrinsic clinical severity by multiplying the average of the assertions Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \bar{\delta_n}} and Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \bar{\gamma_n}} for a Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau=[0|1]} where Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau=0} indicates 'low severity' while Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau=1} 'high severity'.
To summarize in our case 'Bruxer' we therefore have:
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_o\cup ( {\bar\delta_n)} \tau_o + \Im_n\cup({\bar\gamma_n)}\ \tau_n= \Im_d }
where
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle {\bar\delta_n}=} average of the value of clinical statements in the dental context and therefore Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle {\bar\delta_n}=0}
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle {\bar\gamma_n}=} average of the value of clinical statements in the neurological context and therefore Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle {\bar\gamma_n}=0,33}
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau_o=0} reporting of low severity of the dental context
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau_n=1} reporting of high severity of the neurological context
where the 'consistency marker will define the diagnostic path as follows
As can be seen in our clinical case 'Bruxer' we have a very slight diagnostic slope towards the neurological context which allows us, however, to glimpse more of a neurological component rather than a dental one.
Once the myriad of normative data reported positively, which generate conflict between contexts, has been washed away, thanks to the coherence demarcator we have a much clearer and more linear picture on which to deepen the analysis of the functionality of the Central Nervous System ( CNS) than in our clinical case ' Bruxer' appears somewhat intrigued by the low diagnostic weight derived from the neurological assertions .
This average figure derives primarily from a hypothetical jaw jerk amplitude anomaly labeled with an asterisk (*). We will talk about it in the section dedicated to this trigeminal reflex.
Consequently we can concentrate on intercepting the tests necessary to decrypt the machine language code that the CNS sends outwards converted into verbal language which at first sight would seem to concern a sort of hyperreflexia of the tendon reflexes. and specifically the jaw jerk.[9][10][11] To confirm this hypothetical intuition, a brainstorming of the type 'Cognitive Neural Network' abbreviated as 'RNC' presented for the diagnosis of the case of our 'Mary Poppins' in the chapter 'Encrypted code: Ephaptic transmission' is necessary.
However, through this first diagnostic process we have made progress because, contrary to the codified process in dental disciplines, we are undertaking a neurophysiological process to decrypt the machine language code of 'bruxism'.
In order not to weigh down the discussion, we will deal with the second diagnostic step of the Masticationpedia model in the following chapter entitled 'Encrypted code: Hyperexcitability of the trigeminal system'
- ↑ Thompson PD, Obeso JA, Delgado G, Gallego J, Marsden CD. Focal dystonia of the jaw and differential diagnosis of unilateral jaw and masticatory spasm. J Neurol Neurosurg Psychiatry. 1986;49:651–656. doi: 10.1136/jnnp.49.6.651. [PMC free article][PubMed] [CrossRef] [Google Scholar][Ref list]
- ↑ Fernandes G, Franco AL, Gonçalves DA, Speciali JG, Bigal ME, Camparis CM. Temporomandibular disorders, sleep bruxism, and primary headaches are mutually associated. J Orofac Pain. 2013;27(1):14–20. [PubMed] [Google Scholar] [Ref list]
- ↑ Lobbezoo F. Taking up challenges at the interface of wear and tear. J Dent Res. 2007;86(2):101–103. doi: 10.1177/154405910708600201.[PubMed] [CrossRef] [Google Scholar][Ref list]
- ↑ Dubner R, Ren K. Brainstem mechanisms of persistent pain following injury. J Orofac Pain. 2004;18(4):299–305. [PubMed] [Google Scholar] [Ref list]
- ↑ Wang K, Svensson P, Arendt-Nielsen L. Modulation of exteroceptive suppression periods in human jaw-closing muscles by local and remote experimental muscle pain. Pain. 1999;82(3):253–262. doi: 10.1016/S0304-3959(99)00058-5.[PubMed] [CrossRef] [Google Scholar][Ref list]
- ↑ Ro JY, Capra NF. Modulation of jaw muscle spindle afferent activity following intramuscular injections with hypertonic saline. Pain. 2001;92(1–2):117–127.[PubMed] [Google Scholar] [Ref list]
- ↑ Winocur E, Gavish A, Voikovitch M, Emodi-Perlman A, Eli I. Drugs and bruxism: a critical review. J Orofac Pain. 2003;17(2):99–111. [PubMed] [Google Scholar] [Ref list]
- ↑ Katsarava Z, Egelhof T, Kaube H, Diener HC, Limmroth V. Symptomatic migraine and sensitization of trigeminal nociception associated with contralateral pontine cavernoma. Pain. 2003;105(1–2):381–384.[PubMed] [Google Scholar] [Ref list]
- ↑ S Watanabe , H Mochizuki, I Nakashima, Y Itoyama. A case of primary Sjögren's syndrome with CNS disease mimicking chronic progressive multiple sclerosis.Rinsho Shinkeigaku. 1998 Jul;38(7):658-62.
- ↑ Ibrahim M Norlinah, Kailash P Bhatia, Karen Ostergaard, Robin Howard, Gennarina Arabia, Niall P Quinn. Primary lateral sclerosis mimicking atypical parkinsonism. Mov Disord. 2007 Oct.31;22(14):2057-62. doi: 10.1002/mds.21645.
- ↑ M Yoshida, N Murakami, Y Hashizume, A Takahashi. A clinicopathological study on 13 cases of motor neuron disease with dementia.Rinsho Shinkeigaku.1992 Nov;32(11):1193-202.