Hemimasticatory spasm
Hemimasticatory spasm
Abstract: This chapter introduces the complex diagnostic pathway to identify the condition known as "Hemimasticatory Spasm" in the case of a patient referred to as "Mary Poppins." While diagnosing this neuromotor disorder may not be overly complex, differentiating it from other conditions such as Hemifacial Spasm or pathologies of the central and peripheral nervous systems is critical for guiding appropriate treatment.
The chapter begins by examining involuntary movement disorders induced by trauma to cranial or peripheral nerves, referencing studies that highlight the involvement of central nervous system pathologies. Special attention is given to conditions like vestibular and trigeminal schwannomas, as well as other rare cases such as tumors affecting facial and masticatory functions. Further complexity arises when considering multiple sclerosis and pleomorphic adenoma as potential sources of trigeminal reflex anomalies, all of which require careful differentiation.
Additionally, the discussion explores conditions like scleroderma, with particular emphasis on Morphea, which is identified in the patient. The possibility of Morphea-induced Hemimasticatory Spasm is discussed based on trigeminal nerve clinical and electrophysiological findings. The role of electrical excitatory activities, both normal and ephaptic, is considered a significant contributor to abnormal involuntary muscle movements.
The chapter concludes by introducing "ephaptic transmission" as the key to understanding the abnormal communication phenomena in Hemimasticatory Spasm. Ephaptic transmission, a complex form of electrical signaling between neurons, is explored as part of the diagnostic process, providing a foundation for understanding the electrical dynamics underlying the condition. This crucial concept will be examined in greater detail in a subsequent chapter focused on the two forms of electrical transmission between neurons.
Introduction to the Hemimasticatory Spasm
Before getting into the heart of the discussion regarding the pathology of our patient Mary Poppins, what from the previous chapters seems to be of a neuromotry type and in particular a 'Hemimasticatory Spasm' we should focus on some points to determine the process of decryption of the signal.
Let's start by saying that it is not so complex to make a diagnosis of 'Hemimasticatory Spasm' but it is to make a differential diagnosis between 'Hemifacial Spasm' and the nature of the disease to direct the therapy.
We should, first, consider induced movement disorders which can be defined as involuntary or abnormal movements triggered by trauma to the cranial or peripheral nerves or roots.[1] From this it is contextual to consider involuntary movements including spasms, also pathologies of the Central Nervous System as well as the peripheral one. In a study by Seung Hwan Lee et al.[2] two vestibular schwannomas, five meningiomas, and two epidermoid tumors were included. Hemifacial spasm occurred on the same side of the lesion in eight patients while it occurred on the opposite side of the lesion in only one patient. Regarding the pathogenesis of hemifacial spasms, the vessels were found to be involved in six patients, the tumor had involved the lining of the facial nerve in one patient, hypervascular tumor compression of the facial nerve without damage to the vessels in one patient, and a huge tumor which compressed the brainstem with involvement, therefore, of the contralateral facial nerve in one patient. Hemifacial spasm resolved in seven patients, whereas in two patients with a vestibular schwannoma and an epidermoid tumor, it improved transiently and then recurred after one month.
Therefore, keep in mind localizations, including central ones, which could cause facial and/or masticatory spasm, for example, cases of vestibular schwannoma and epidermal tumor.
Vestibular and trigeminal schwannoma
Secondary hemifacial spasm due to vestibular schwannoma is very rare. The study by S Peker et al.[3] was the first reported case of hemifacial spasm responsive to gamma knife radiosurgery in a patient with an intracanalicular vestibular schwannoma. Both spasm resolution and tumor growth control were achieved with a single session of gamma knife radiosurgery. The 49-year-old male patient with a 6-month history of right-sided hearing loss and hemifacial spasm. MRI examination revealed an intracanalicular vestibular schwannoma. The patient was treated with radiosurgery and received 13 Gy at the 50% isodose line. Control of tumor growth was achieved and there was no change in tumor volume at the latest follow-up at 22 months. The hemifacial spasm completely resolved after one year. Surgical removal of the presumably causative mass lesion has been reported to be the only treatment in secondary hemifacial spasm.
MRI is the imaging modality of choice and is usually diagnostic in the appropriate clinical setting. The thin T2-weighted 3D CISS axial sequence is important for correct evaluation of the cisternal segment of the nerve. They are usually hypointense on T1, hyperintense on T2 with enhancement after gadolinium. But we cannot be surprised if cases like the one described by Brandon Emilio Bertot et al[4] occur. in which a clinical case of a 16-year-old boy with an atypical incidence of a large trigeminal schwannoma presenting with painless malocclusion and unilateral masticatory weakness was presented. This case is the first documented case, to our knowledge, in which a trigeminal schwannoma generated a true malocclusion with masseter weakness and is the 19th documented case of unilateral trigeminal motor neuropathy of various etiology. From a study by Ajay Agarwal,[5] however, it is clear that intracranial trigeminal schwannomas are rare tumors. Patients usually present with symptoms of trigeminal nerve dysfunction, the most common symptom being facial pain.
Multiple sclerosis and trigeminal reflexes
We must make a further premise regarding axonal demyelination in multiple sclerosis. A study by Joanna Kamińska et el.[6] demonstrated that multiple sclerosis (MS) is a chronic inflammatory and demyelinating disease of autoimmune origin. The main agents responsible for the development of MS include exogenous, environmental and genetic factors. MS is characterized by multifocal and temporally scattered damage to the Central Nervous System (CNS) leading to axonal damage. Among the clinical courses of MS we can distinguish relapsing-remitting multiple sclerosis (RRMS), secondary progressive multiple sclerosis (SPSM), primary progressive multiple sclerosis (PPMS) and relapsing progressive multiple sclerosis (RPMS). Depending on the severity of the signs and symptoms, MS can be described as benign MS or malignant MS. The diagnosis of MS is based on the McDonald's diagnostic criteria, which link the clinical manifestation with the characteristic lesions demonstrated by magnetic resonance imaging (MRI); by analysis of cerebrospinal fluid (CSF) and visual evoked potentials. It should be emphasized that, despite enormous progress regarding MS and the availability of different diagnostic methods, this disease still represents a diagnostic challenge. It may result from the fact that MS has a different clinical course and lacks a single test of appropriate diagnostic sensitivity and specificity for rapid and accurate diagnosis. Precisely in reference to this last observation we must point out another significant data that emerged from a study by S K Yates and W F Brown[7] in which we read that the jaw jerk of the masseter is present in all control subjects but commonly absent in patients with sclerosis defined multiple (SM). In some MS patients the latency was prolonged. Jaw jerk abnormalities, however, are less frequent than blink reflex responses to supraorbital nerve stimulation. However, there have been patients in whom the blink reflex was normal but the jaw jerk responses were abnormal.
The latter observation suggests that the jaw jerk may occasionally be useful in the detection of brainstem lesions in MS.
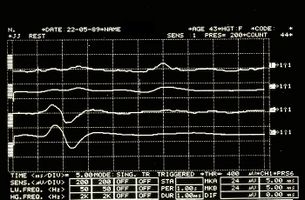
But at this point the doubt becomes reality: what should we think, then, of the anomalies of the trigeminal reflexes that emerged in our Mary Poppins? Could we be facing a form of 'Multiple Sclerosis? How do we distinguish the location of any demyenization whether Central or Peripheral? (Figure 3 and 4)
Pleomorphic adenoma
Pleomorphic adenoma is a common benign neoplasm of the salivary glands characterized by neoplastic proliferation of epithelial (ductal) cells together with myoepithelial components, with malignant potential. It is the most common type of salivary gland tumor and the most common tumor of the parotid gland. It derives its name from architectural pleomorphism (variable appearance) seen under an optical microscope. It is also known as a "mixed tumor, salivary gland type", which refers to its dual origin from epithelial and myoepithelial elements in contrast to its pleomorphic appearance.
The diagnosis of salivary gland tumors uses both tissue sampling and radiographic studies. Tissue sampling procedures include fine needle aspiration (FNA) and core needle biopsy (larger needle than FNA). Both of these procedures can be performed on an outpatient basis. Diagnostic imaging techniques for salivary gland tumors include ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI). CT allows direct bilateral visualization of the salivary gland tumor and provides information on overall size and tissue invasion. CT is excellent for demonstrating bony invasion. MRI provides superior delineation of soft tissues such as perineural invasion compared to CT alone as well described by Mehmet Koyuncu et al.[8]

This last observation is very important because an invasion of the tumor of the nervous tissues in the infratemporal fossa cannot be excluded and precisely because of the complexity of the disease, we report a work by Rosalie A Machado et al.[9], which can be explored in depth in the sub-chapter of Masticationpedia 'Intermittent facial spasms as the presenting sign of a recurrent pleomorphic adenoma' in which the authors confirm that to date the development of facial spasms has not been reported in parotid neoplasms. The most common etiologies for hemifacial spasm are vascular compression of the facial nerve ipsilateral to the cerebellopontine angle (defined as primary or idiopathic) (62%), hereditary (2%), secondary to Bell's palsy or facial nerve injury (17 %) and imitators of hemifacial spasms (psychogenic, tics, dystonia, myoclonus, myokymia, myorrhythmia and hemimasticatory spasm) (17%).
Scleroderma
Tiago Nardi Amaral et al.[10] described the clinical characteristics, neuroimaging, and treatment of neurological involvement in systemic sclerosis (SSc) and localized scleroderma (LS) through a systematic review
The authors carried out a literature search in PubMed using the following MeSH terms, scleroderma, systemic sclerosis, localized scleroderma, localized scleroderma "en coup de sabre", Parry-Romberg syndrome, cognitive impairment, memory, seizures, epilepsy, headache , depression, anxiety, mood disorders, Center for Epidemiological Studies in Depression (CES-D), SF-36, Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI), Patient Health Questionnaire-9 (PHQ-9 ), neuropsychiatry, psychosis, neurological involvement, neuropathy, peripheral nerves, cranial nerves, carpal tunnel syndrome, ulnar entrapment, tarsal tunnel syndrome, mononeuropathy, polyneuropathy, radiculopathy, myelopathy, autonomic nervous system, nervous system, electroencephalography (EEG), electromyography (EMG), magnetic resonance imaging (MRI), and magnetic resonance angiography (MRA). Patients with other connective tissue diseases responsible for nervous system involvement were excluded from the analyses.
A total of 182 case reports/studies addressing SSc and 50 reporting LS were identified. The total number of patients with SSc was 9,506, while data were available on 224 patients with LS. In LS, convulsions (41.58%) and headache (18.81%) predominated. However, descriptions of various cranial nerve involvement and hemiparesis have been made. Central Nervous System involvement in SSc was characterized by headache (23.73%), seizures (13.56%), and cognitive impairment (8.47%). Depression and anxiety were frequently observed (73.15% and 23.95%, respectively). Myopathy (51.8%), trigeminal neuropathy (16.52%), peripheral sensorimotor polyneuropathy (14.25%), and carpal tunnel syndrome (6.56%) were the most frequent peripheral nervous system involvement in SSc. Autonomic neuropathy involving the cardiovascular and gastrointestinal systems has been regularly described. The treatment of nervous system involvement, however, varied from case to case. However, in more severe cases corticosteroids and cyclophosphamide were usually prescribed.
But this is not all because there are some variants of scleroderma such as Morphea diagnosed in our poor patient Mary Poppins who among other things did not respond positively to cortisone therapy.
Morphea
Morphea is a form of scleroderma that involves isolated patches of hardened skin on the face, hands and feet, or anywhere else on the body, without involvement of internal organs. Morphea most often presents as macules or plaques a few centimeters in diameter, but can also present as bands or in guttate lesions or nodules.[11] Morphea is a thickening and hardening of the skin and subcutaneous tissues due to excessive collagen deposition . Morphea encompasses specific conditions ranging from very small plaques involving only the skin to widespread disease causing functional and cosmetic deformities. Morphea is distinguished from systemic sclerosis by its presumed lack of involvement of internal organs.[12]
Unfortunately the path is still difficult because the long series of variants does not exclude a form of Morphea-induced hemimasticatory spasm as well described by H J Kim et al.[13] in which it is asserted that on the basis of trigeminal clinical and electrophysiological findings such as the blink reflex, the jaw jerk and the masseteric silent period, focal demyelination of the motor branches of the trigeminal nerve due to deep tissue alterations is suggested as a cause of electrical activities abnormal excitatory movements resulting in involuntary chewing movement and spasm.
The latter assertion indicates an involvement of normal and ephaptic excitatory electrical activities.
Conclusion
Before discussing the paths implemented to reach the diagnosis of 'Hemimasticatory spasm' of our poor patient Mary Poppins, we should anticipate that the encrypted code that we were trying to identify as a communication phenomenon concerns 'ephaptic transmission', a very important and complex phenomenon to evoke but above all requires a description of the electrical transmission between neurons.
Electrical signaling is a key feature of the nervous system and gives it the ability to react quickly to changes in the environment. Although synaptic communication between nerve cells is primarily perceived as chemically mediated, electrical synaptic interactions also occur. Two different strategies are responsible for electrical communication between neurons. One is the consequence of low-resistance intercellular pathways, called “gap junctions,” for the diffusion of electrical currents between the inside of two cells. The second occurs in the absence of cell-cell contacts and is a consequence of the extracellular electric fields generated by the electrical activity of neurons. In the chapter dedicated to this fundamental topic, current notions on electrical transmission will be discussed in a historical perspective, comparing the contributions of the two different forms of electrical communication to brain function. ( see Two Forms of Electrical Transmission Between Neurons*)
- ↑ Joseph Jankovic. Peripherally induced movement disorders Neurol Clin. 2009 Aug;27(3):821-32, vii, doi: 10.1016/j.ncl.2009.04.005.
- ↑ Seung Hwan Lee 1, Bong Arm Rhee, Seok Keun Choi, Jun Seok Koh, Young Jin Lim. Cerebellopontine angle tumors causing hemifacial spasm: types, incidence, and mechanism in nine reported cases and literature review. Acta Neurochir (Wien) 2010 Nov;152(11):1901-8. doi: 10.1007/s00701-010-0796-1.Epub 2010 Sep 16.
- ↑ S Peker, K Ozduman, T Kiliç, M N Pamir. Relief of hemifacial spasm after radiosurgery for intracanalicular vestibular schwannoma. Minim Invasive Neurosurg. 2004 Aug;47(4):235-7. doi: 10.1055/s-2004-818485.
- ↑ Brandon Emilio Bertot, Melissa Lo Presti, Katie Stormes, Jeffrey S Raskin, Andrew Jea, Daniel Chelius, Sandi Lam. Trigeminal schwannoma presenting with malocclusion: A case report and review of the literature.Surg Neurol Int. 2020 Aug 8;11:230. doi: 10.25259/SNI_482_2019.eCollection 2020.
- ↑ Ajay Agarwal. Intracranial trigeminal schwannoma Ajay Agarwal. Neuroradiol J.2015 Feb;28(1):36-41. doi: 10.15274/NRJ-2014-10117.
- ↑ Joanna Kamińska, Olga M Koper, Kinga Piechal, Halina Kemona . Multiple sclerosis - etiology and diagnostic potential.Postepy Hig Med Dosw. 2017 Jun 30;71(0):551-563.doi: 10.5604/01.3001.0010.3836.
- ↑ S K Yates, W F Brown. The human jaw jerk: electrophysiologic methods to measure the latency, normal values, and changes in multiple sclerosis.Neurology. 1981 May;31(5):632-4.doi: 10.1212/wnl.31.5.632.
- ↑ Mehmet Koyuncu, Teoman Seşen, Hüseyin Akan, Ahmet A Ismailoglu, Yücel Tanyeri, Atilla Tekat, Recep Unal, Lütfi Incesu. Comparison of computed tomography and magnetic resonance imaging in the diagnosis of parotid tumors.Otolaryngol Head Neck Surg. 2003 Dec;129(6):726-32.doi: 10.1016/j.otohns.2003.07.009.
- ↑ . 2017 Feb 10;8(1):86-90. doi: 10.5306/wjco.v8.i1.86. Rosalie A Machado, Sami P Moubayed, Azita Khorsandi, Juan C Hernandez-Prera, Mark L Urken. Intermittent facial spasms as the presenting sign of a recurrent pleomorphic adenoma. World J Clin Oncol. 2017 Feb 10;8(1):86-90. doi: 10.5306/wjco.v8.i1.86.
- ↑ Tiago Nardi Amaral, Fernando Augusto Peres, Aline Tamires Lapa, João Francisco Marques-Neto, Simone Appenzeller. Neurologic involvement in scleroderma: a systematic review Semin Arthritis Rheum. 2013 Dec;43(3):335-47. doi: 10.1016/ j.semarthrit. 2013.05.002. Epub 2013 Jul 1.
- ↑ James, William; Berger, Timothy; Elston, Dirk (2005). Andrews' Diseases of the Skin: Clinical Dermatology. (10th ed.). Saunders. Page 171. ISBN 0-7216-2921-0.
- ↑ James, William; Berger, Timothy; Elston, Dirk (2005). Andrews' Diseases of the Skin: Clinical Dermatology. (10th ed.). Saunders. Page 171. ISBN 0-7216-2921-0.
- ↑ H J Kim, B S Jeon, K W Lee. Hemimasticatory spasm associated with localized scleroderma and facial hemiatrophy.Arch Neurol. 2000 Apr;57(4):576-80. doi: 10.1001/archneur.57.4.576.