7° Clinical case: Brainstem neoplasm in Orofacial pain
7° Clinical case: Brainstem neoplasm in Orofacial pain
Abstract:The brainstem, a crucial structure connecting the brain to the spinal cord, plays a vital role in sensory, motor, and autonomic functions. This chapter explores a clinical case from 1995 involving a 60-year-old woman, pseudonymously named 'Capsaicin,' who presented with chronic orofacial pain, burning mouth syndrome (BMS), and a history of maxillofacial prosthetic rehabilitation. Initial gnathological and neurological tests did not reveal significant abnormalities, leading to diagnostic uncertainty. However, persistent symptoms, exacerbated by spicy food, raised suspicion of a deeper neurological pathology. The application of the "Coherence Demarcator" model failed to identify a definitive diagnosis, prompting further investigation.
Subsequent MRI scans revealed a brainstem schwannoma, confirming the presence of severe organic neurological damage. Additionally, the involvement of TRPV1 (Transient Receptor Potential Vanilloid 1) channels was highlighted as a possible link between capsaicin-induced pain and neuroinflammation. This case underscores the importance of considering neuro-immune interactions and the limitations of traditional diagnostic frameworks such as the RDC/TMD when addressing complex orofacial pain.
The case emphasizes the need for a more integrated diagnostic approach, one that accounts for rare neurological conditions like brainstem tumors, and suggests that future research focus on TRPV1’s role in pain modulation and neuroinflammation. Capsaicin's paradoxical effect, both as a pain exacerbator and potential therapeutic agent, serves as a reminder of the intricacies in understanding chronic pain conditions.
Introduction
The brain stem is the caudal portion of the brain that connects the diencephalon to the spinal cord and cerebellum.[1] The brainstem mediates the sensory and motor pathways between the spinal cord and the brain and contains the nuclei of the cranial nerves, the ascending reticular activating system (ARAS), and the autonomic nuclei. It controls brainstem reflexes and the sleep-wake cycle and is responsible for autonomous control of the cardiovascular, respiratory, digestive and immune systems. Brainstem dysfunction can result from various acute or chronic insults, including stroke, infectious, cancer, inflammatory, and neurodegenerative diseases. In the context of critical illness, the brain stem can be susceptible to various insults that can be classified as structural and non-structural in origin. Brainstem dysfunction can therefore contribute to impaired consciousness, cardiocirculatory and respiratory insufficiency and therefore to increased mortality [2][3][4][5] and especially manifest as orofacial pain (OP).Brainstem dysfunction in critically ill patients:
These important premises extracted from an interesting article by Sarah Benghanem[6] are essential details, expression of a clinical experience which led the author of the chapter to scientific and epistemological reflections on the typology of language to be used in the creation of diagnostic models. and consequently to found Masticationpedia. One cannot, personal and responsible assertion of the author of the chapters, slavishly follow an axiom, a protocol such as the DRC or whatever and risk an error of differential diagnosis which can cost the life of a human being. If there is a gap in the model, which we will demonstrate during the implementation of Masticationpedia, then it should be noted as an anomaly, analyzed and eliminated or at least modified otherwise it is not a question of paradigmatic progress but only of intensive progress.
Presentation of the clinical case
It was the year 1995 when the 60-year-old female patient, who we will call with a fancy name 'Capsaicin' (we will understand the reason below) presented herself to our observation reporting bilateral diffuse orofacial pain in the temporal muscles region and in the occipital region , in addition, to the presence of burning mouth (BMS), for more than 10 years. The pain lasted for hours especially at night and did not occur cyclically. The patient was the bearer of a long-standing upper and lower ceramic fixed prosthetic rehabilitation with various fractures of the ceramic structure and wear veneers on the remaining natural teeth. However, no occlusal discrepancies were found in the prosthetic rehabilitation, but a release plate was performed by other health professionals to be used at night. The patient reported pain even with the plate inserted and was initially considered to be affected by Atypical Orofacial Pain (AOP) with a strong psychosomatic component and subsequently, according to the RDC protocol, affected by 'Temporomandibular Disorders'.
Having come to our observation, as per the Masticationpedia protocol, the main gnathological tests were performed, such as paraocclusal axiography of the condylar tracings and an interferential EMG of the masseter muscles. In this case, an ATM CT was not requested, much less an MR. We present in a schematic and representative way reporting some paragraphs exposed in the previous chapters the Masticationpedia protocol in its schematization as: assertions in the dental, neurological context and finally the diagnostic conclusion through the 'Demarcator Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau} ':
Significance of contexts
For the dental context we will have the following sentences and statements to which we give a numerical value to facilitate the treatment and that is Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_n=[0|1]} where Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_n=0} indicates 'normality' and Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_n=1} 'abnormality and therefore positivity of the report:
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_1=} Paraocclusal axiographic tracing of the right condyle, negative in Figure 1, Normality, negative report
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_2=} Paraocclusal axiography tracing of the left condyle, negative in Figure 2, Normality, negative report
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_3=} Symmetric EMG interference pattern in Figure 5. Normality, negative report
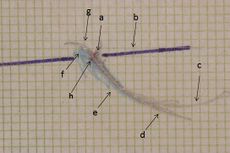
Figure 1: Right consular paraocclusal axiographic tracing: a) Hinge axis center, b) orbital axis plane, c) protrusive tracing, d) mediotrusive tracing, e) mediotrusive masticatory cycle area, f) laterotrusive masticatory cycle area, g) laterotrusive backline , h) consular hinge axis in maximum intercuspidation,
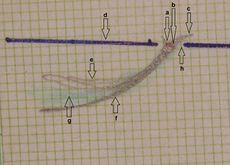
Figure 2: Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_2} Right consular paraocclusal axiographic tracing: a) Hinge axis center, b) orbital axis plane, c) protrusive tracing, d) mediotrusive tracing, e) mediotrusive masticatory cycle area, f) laterotrusive masticatory cycle area, g) laterotrusive backline.
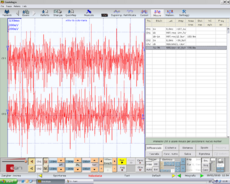
Figura 3: Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_3} Interferential EMG of right (top) and left (bottom) masseter muscles. Amplitude 200μV per division, time division 100 msec
The sentence Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_o} (dental context) with a number Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle n\geq1} of other logically compatible statements Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle (\delta_1,\delta_2,.....\delta_n \ )} determine the union and coherence between them Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_o\cup\{\delta_1,\delta_2.....\delta_n\}} and are represented with a mathematical formalism to facilitate the diagnostic dialectic in the following way Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_o\cup\{\delta_1,\delta_2.....\delta_n\}\rightarrow \Im_o\cup\{0,0.....0\}=0} which represents the average of the individual assertions reported. The average has been designed because it can often happen that some tests give answers with negative reporting to which to give the value Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_n=0} . The result in this case is Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_n=0} and contextually derives the coherent affirmation of the sentence in which it is argued that the symptomatology of the 'Capsaicin' patient is not determined by the presence of a TMDs.
In the neurological context we will therefore have the following sentences and assertions to which we obviously give, as for the dental context, a numerical value to facilitate the treatment and that is Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_n=[0|1]} where iFailed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_n=0} indicates 'normality' and Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_n=1} 'abnormality' and therefore positivity of the report:
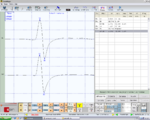
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_1=} bRoot-MEPs symmetrical in amplitude and latency in Figure 4, Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_1=0\longrightarrow} Normality, negative report
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_2=} Jaw jerk symmetrical in amplitude and in latency in Figure 8, Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_2=0\longrightarrow} Normality, negative report
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_3=} Symmetry in duration of the masseter silent period in Figure 6, Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_3=0\longrightarrow} Normality, negative report
Consequently even in sentence Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_n} with a number Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle n\geq1} of other logically compatible assertions they determine the union and coherence between them Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_n\cup\{\gamma_1,\gamma_2.....\gamma_n\}} and the formal representation will be similar to that of the dental context. Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_n\cup\{\gamma_1,\gamma_2.....\gamma_n\}\rightarrow \Im_n\cup\{0,0.....0\}=0} with contextual coherent affirmation of the sentence Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_n} in which it is argued that the symptomatology of the patient 'Capsiacin' does not electrophysiologically highlight any element that could indicate a correlation between orofacial pain and neuromotor damage.
Coherence Demarcator Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau}
The Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau} is a representative clinical specific weight, complex to research and develop because it varies from discipline to discipline and for pathologies, essential for not colliding logical assertions Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_o} and Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_n} in diagnostic procedures and fundamental for initializing the decryption of the machine code. Basically it allows to confirm the coherence of one union Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im\cup\{\delta_1,\delta_2.....\delta_n\}} with respect to another Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im\cup\{\gamma_1,\gamma_2.....\gamma_n\}} and vice versa, giving greater weight to the seriousness of the assertions and of the report in the appropriate context. Sometimes the doctor is faced with a series of positive reports to which he must give the right weight, he must consider the positivity of a report which highlights, for example, an osteoarticular renewal of the TMJ cannot have the same weight as a positivity of a report confirming latency delay of a trigeminal reflex. The demarcation Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau} weight, therefore, gives more significance to the more serious assertions in the clinical context from which they derive and therefore beyond the positivity of the assertions Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \delta_n=1} or which Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \gamma_n=1} in any case are always verified and respected, these must be multiplied by a Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau=[0|1]} where Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau=0} indicates 'Low seriousness ' while Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tau=1} 'Severe Gravity'.
A consistency marker could be defined as a real number between 0 and 1 in the event that we have more than two reports and therefore a number close to zero would correspond to a report of 'low severity' while a number close to one to a reporting of 'severe severity'. But this is redundant. What is done is to compare the reports two by two and then use the values we have indicated to express the 'Gravity'.
So to recap we have:
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Im_o\cup ( {\bar\delta_n)} \tau_o + \Im_n\cup({\bar\gamma_n)}\ \tau_n= \Im_d }
where
average of the value of the clinical statements in the dental context which in our case in question is
average of the value of clinical statements in the neurological context and therefore
reporting of low severity of the dental context
reporting of low severity of the neurological context
where the 'demarcator of coherence marker' will define the diagnostic path as follows
As we will see during the exposition of the clinical cases in Masticationpeida, one will be faced with similar clinical situations in which you are in the presence of orofacial pain but the reporting results in specialist contexts will be of low severity such as to cancel the discriminate value of .
(The fact remains that the patient continues to experience orofacial pain and particularly with exacerbation after a spicy dinner.)
In the rare but real clinical cases in which the ' Demarcator' is reset, we are motivated to carry out an even more extensive and in-depth check, hypothesizing serious pathologies [7][8] including head and neck tumors which simulate symptoms that can be superimposed on other pathologies. It is a serious mistake to consider the patient with such clinical manifestations and the simultaneous absence of systemic anomalies as a patient with psychosomatic disorders. Elements of psychophysical damage can certainly coexist but, if the 'Demarcator' fails, it is mandatory to deepen the diagnostics. In fact, head and neck cancer (HNC) affects over 890,000 people each year worldwide and has a 50% mortality rate. Aside from poor survival, HNC pain impairs eating, drinking and speaking, severely reducing quality of life. The different pain phenotype in patients (allodynia, hyperalgesia, and spontaneous pain) results from a combination of anatomical, histopathological, and molecular differences between tumors[9]. Glial and immune modulation of the tumor microenvironment, as well explained in the article by Ye et al., influences not only cancer progression but also pain signaling among which transient receptor potentials contained in gustatory somatosensory systems are an example.[10][11]
For the above reasons and for the persistence of the OP, the difficulty in concluding a certain diagnosis, the absence of organic-functional discrepancies and the zeroing of the ' Demarcator', an MR of the brain was requested.
Imaging showed a neoplastic mass most likely a brainstem schwannoma with invagination of the occipital frame. (figure 7 and 8)
(.......leaving a doubt, that of the correlation between pain and capsaicin as reported by the patient on the occasion of a spicy diet.)
Thoughts and conclusions
The mammalian gustatory system is made up of taste buds, which are clusters of 50-100 taste cells found throughout the oral cavity. On the tongue, which is the central topic of the case report 'Capsaicin', the taste buds are located on circumvallate, foliate and fungiform papillae. Taste cells synapse with afferent fibers from branches of the cranial facial (CN VII), glossopharyngeal (CN IX) and vagus (CN X) nerves which, in turn, transmit information to the central nervous system (CNS) about gustatory attributes , intensity and hedonic nature. [12][13][14] The taste buds are embedded in a stratified squamous epithelium, which contains somatosensory branches of the trigeminal (CN V), glossopharyngeal (CN IX), and vagus (CN X) cranial nerves. Information from these general sensory nerves provides information to the central nervous system about mechanical, thermal, and pain stimuli.[11][15] Painful stimuli can result from strong or sharp mechanical stimuli, abnormally high or low temperatures, or chemical stimuli such as capsaicin, which is found in hot peppers and causes a burning taste sensation through the intervention of Transient Receptor Potentials (TRPs).[10] These TRPs are divided into six subfamilies including TRPV1, which we are interested in to hypothesize the phenomenon of pain exacerbation of the patient 'Capsaicin' in spicy diet.

TRPV1 and neuroinflammation
TRPV1s constitute a distinct subset of non-selective cation channels (Transient Receptor Potential) responsible for many cellular responses. They are activated by various stimuli such as acids, extracellular protons, high temperatures, plant toxins and vanilloid agonists. The TRPV1s present in mammals can be considered as sensors of chemical substances (capsaicin), thermal substances (heat) and/or harmful stimuli. The activation of TRPV1 leads to the depolarization necessary for the propagation of action potentials along the axons of the dorsal root ganglia (DRG) of neurons that project to the spinal cord and consequently also to the nociceptive trigeminal nuclei. What makes TRPV1 so critical for pain signaling is undeniably its ability to transduce inflammatory signals into electrical signals with the activation of both voltage-gated sodium and calcium channels located at the nociceptor level.[16] The implication of TRPV1 in pathological pain prompted a careful study of these proteins. The limiting element in pharmacological research at the application level was the peculiarity of the TRPV1 channel, i.e. its polymodal mechanism of activation (heat, capsaicin, pH), which led to a high level of complexity in the design of a specific modality inhibitor.
The interaction between neurons and immune cells is a well-known phenomenon.[17] TRPV1 plays its role, too, in neuroinflammation by being expressed in both neurons and immune cells. Significant importance should be given to the confirmed expression of TRPV1 in microglia and astrocytes, cells found in the vicinity of neurons. The neuro-immune axis is the site of production of neuroinflammatory molecules and receptors that interact between the two systems and ensure a complex response to external stimuli (or to the body's own pathologies). TRPV1 is said to contribute to microglia autophagy through its Ca2+ signaling, which leads to mitochondria-induced cell death. Basically, TRPV1 is a pro-apoptotic element.
Ligands
Antagonists
Antagonists block the activity of TRPV1, thereby reducing pain. Antagonists identified include the competitive antagonist capsazepine and the non-competitive antagonist ruthenium red. These agents could be beneficial if applied systematically.[18] TRPV1 antagonists have shown efficacy in reducing nociception from inflammatory and neuropathic pain models in rats.[19] This provides evidence that TRPV1 is the sole receptor for capsaicin.[20] In humans, drugs that target TRPV1 receptors could be used to treat neuropathic pain associated with multiple sclerosis, chemotherapy, or amputation, as well as pain associated with the inflammatory response of damaged tissue, such as in osteoarthritis.[21]
Agonists
TRPV1 is activated by several agonists of natural origin.[22] Agonists such as capsaicin and resiniferatoxin activate TRPV1 and, after prolonged application, cause the decrease of TRPV1 activity (desensitization), leading to pain relief through the subsequent decrease of the TRPV1-mediated release of inflammatory molecules upon exposure to noxious stimuli.
An interesting study by the Tominaga group extends the list of TRPV1 interactions also to Anoctamin 1 (ANO 1) also known as Transmembrane member 16A (TMEM16A) a chloride channel which is usually activated by Ca2+. The authors demonstrate, in fact, that when TRPV1 interacts with the ANO1 channel it mediates the efflux of Chloride evoking depolarization (after stimulation by capsaicin) with increased excitability of the nociceptor. Tominaga highlighted a clear structural and functional crosstalk between TRPV1 and ANO1, which intervenes in the algogenic action of capsaicin.
These results demonstrate the importance of chloride homeostasis in the regulation of the excitability of the neuronal DRGs, i.e. of the dorsal root ganglia and obviously of the trigeminal somatosensory nuclei and in the pain phenomenon as a whole; one of the new approaches, therefore, where to intervene to mitigate painful hypersensitivity and neurogenic inflammation.
TRPV1 is activated by several agonists of natural origin. Agonists such as capsaicin and resiniferatoxin activate TRPV1 and, after prolonged application, cause the decrease of TRPV1 activity (desensitization), leading to pain relief through the subsequent decrease of TRPV1-mediated release of inflammatory molecules following administration. exposure to noxious stimuli.
An interesting study by the Tominaga group extends the list of TRPV1 interactions also to Anoctamin 1 (ANO 1) also known as Transmembrane member 16A (TMEM16A)[23] a chloride channel which is usually activated by Ca2+. The authors demonstrate, in fact, that when TRPV1 interacts with the ANO1 channel it mediates the efflux of Chloride evoking depolarization (after stimulation by capsaicin) with increased excitability of the nociceptor. Tominaga[23] highlighted a clear structural and functional crosstalk between TRPV1 and ANO1, which intervenes in the algogenic action of capsaicin.
These results demonstrate the importance of chloride homeostasis in the regulation of the excitability of the neuronal DRGs, i.e. of the dorsal root ganglia and obviously of the trigeminal somatosensory nuclei and in the pain phenomenon as a whole; one of the new approaches, therefore, where to intervene to mitigate painful hypersensitivity and neurogenic inflammation.
(....for chronic damage to the brainstem nerve fibers with consequent alteration of the neuro-immune homeostasis and contextual paradoxical effect of the analgesic action of capsaicin.)
In these cases, as stated in other chapters, we are dealing with an epistemological profile in which the basic knowledge, what we have called (Knowledge Base) corresponds to a temporal limit of scientific information and consequently serious difficulties of differential diagnosis. We had to wait 12 years to reach an ezipathogenetic conclusion overwritten from 1995, a period in which the patient 'Capsaicin' was being treated, to 2007 in which a research gave the elements to suspect the presence of an organic neurological damage with manifestations of pain and burning mouth.[24] Burning mouth syndrome (BMS) is often an idiopathic, chronic intractable pain condition, affecting 1.5-5.5% of middle-aged and older women. We investigated heat and capsaicin receptor TRPV1, and its regulatory nerve growth factor (NGF), in BMS. BMS patients (n=10) and controls (n=10) were evaluated for baseline and post-topical capsaicin pain scores, and their tongue biopsies were immunostained for TRPV1, NGF, and neurofilament structural nerve markers and periphery. Nerve fibers penetrating the epithelium were less abundant in the BMS (p<0.0001), indicating small fiber neuropathy. TRPV1-positive fibers were overall significantly increased in BMS (p=0.0011), as were NGF fibers (p<0.0001) and NGF staining of basal epithelial cells (p<0.0147). There was a significant correlation between baseline pain score and TRPV1 (p=0.0143) and NGF (p=0.0252) fibers. A significant correlation was observed between baseline and post-capsaicin pain (p=0.0006).
- ↑ Hurley RA, Flashman LA, Chow TW, Taber KH. The brainstem: anatomy, assessment, and clinical syndromes. J Neuropsychiatry Clin Neurosci. 2010;22(1):iv. doi: 10.1176/jnp.2010.22.1.iv.
- ↑ Annane D, Trabold F, Sharshar T, Jarrin I, Blanc AS, Raphael JC, et al. Inappropriate sympathetic activation at onset of septic shock: a spectral analysis approach. Am J Respir Crit Care Med août. 1999;160(2):458–465. doi: 10.1164/ajrccm.160.2.9810073.
- ↑ Sharshar T, Porcher R, Siami S, Rohaut B, Bailly-Salin J, Hopkinson NS, et al. Brainstem responses can predict death and delirium in sedated patients in intensive care unit. Crit Care Med août. 2011;39(8):1960–1967. doi: 10.1097/CCM.0b013e31821b843b.
- ↑ Sharshar T, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A, et al. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet Lond Engl. 2003;362(9398):1799–1805. doi: 10.1016/S0140-6736(03)14899-4.
- ↑ Mazeraud A, Pascal Q, Verdonk F, Heming N, Chrétien F, Sharshar T. Neuroanatomy and physiology of brain dysfunction in sepsis. Clin Chest Med. 2016;37(2):333–345. doi: 10.1016/j.ccm.2016.01.013.
- ↑ Benghanem S, Mazeraud A, Azabou E, Chhor V, Shinotsuka CR, Claassen J, Rohaut B, Sharshar T. Brainstem dysfunction in critically ill patients. Crit Care. 2020 Jan 6;24(1):5. doi: 10.1186/s13054-019-2718-9.PMID: 31907011
- ↑ Chloé Gibeili, Arek Sulukdjian, Audrey Chanlon, Nathan Moreau. Unilateral glossodynia as a harbinger of an occult cerebellopontine angle tumour. BMJ Case Report.. 2022 Apr 12;15(4):e249408.doi: 10.1136/bcr-2022-249408.
- ↑ Irappa Madabhavi, Malay Sarkar, K S Sandeep, Mitul Modi. Isolated trigeminal neuralgia: An early weird presentation of carcinoma breast. J Cancer Res Ther. . 2022 Oct-Dec;18(6):1820-1822.doi: 10.4103/jcrt.JCRT_712_20.
- ↑ e Y, Jensen DD, Viet CT, Pan HL, Campana WM, Amit M, Boada MD.Advances in Head and Neck Cancer Pain.YJ Dent Res. 2022 Aug;101(9):1025-1033. doi: 10.1177/00220345221088527. Epub 2022 Apr 13.PMID: 35416080
- ↑ 10.0 10.1 Ramsey, I.S., M. Delling, and D.E. Clapham. 2006. An introduction to TRP channels. Annu Rev Physiol, 68: 619–647.
- ↑ 11.0 11.1 Julius, D. 2013. TRP channels and pain. Annu Rev Cell Dev Biol, 29: 355–584.
- ↑ Gutierrez, R., and S.A. Simon. 2011. Chemosensory processing in the taste-reward pathway. Flavour Fragr J, 26(4): 231–238.
- ↑ Carleton, A., R. Accolla, and S.A. Simon. 2010. Coding in the mammalian gustatory system. Trends Neurosci, 33(7): 326–334.
- ↑ Vincis, R. and A. Fontanini. 2016. A gustocentric perspective to understanding primary sensory cortices. Curr Opin Neurobiol, 40: 118–124
- ↑ Kaneko, Y., and A. Szallasi. 2014. Transient receptor potential (TRP) channels: A clinical perspective. Br J Pharmacol, 171(10): 2474–2507.
- ↑ Bourinet E, Altier C, Hildebrand M E, Trang T, Salter MW, Zamponi GW. Calcium permeable ion channels in pain signaling. Physiol Rev 2014; 94: 81–140.
- ↑ Jacobson A, Yang D, Vella M, Chiu IM (May 2021). "The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes". Mucosal Immunology. 14 (3): 555–565. doi:10.1038/s41385-020-00368-1. PMC 8075967. PMID 33542493.
- ↑ Khairatkar-Joshi N, Szallasi A (January 2009). "TRPV1 antagonists: the challenges for therapeutic targeting". Trends in Molecular Medicine. 15 (1): 14–22. doi:10.1016/j.molmed.2008.11.004. PMID 19097938.
- ↑ Jhaveri MD, Elmes SJ, Kendall DA, Chapman V (July 2005). "Inhibition of peripheral vanilloid TRPV1 receptors reduces noxious heat-evoked responses of dorsal horn neurons in naïve, carrageenan-inflamed and neuropathic rats". The European Journal of Neuroscience. 22 (2): 361–370. doi:10.1111/j.1460-9568.2005.04227.x. PMID 16045489. S2CID 24664751.
- ↑ Story GM, Crus-Orengo L (2008). "Feel the Burn". American Scientist. 95 (4): 326–333. doi:10.1511/2007.66.326. ISSN 0003-0996. Archived from the original on January 19, 2008.
- ↑ Gunthorpe MJ, Szallasi A (2008). "Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms". Current Pharmaceutical Design. 14 (1): 32–41. doi:10.2174/138161208783330754. PMID 18220816.
- ↑ Boonen, Brett; Startek, Justyna B.; Talavera, Karel (2016-01-01). Chemical Activation of Sensory TRP Channels. Topics in Medicinal Chemistry. Springer Berlin Heidelberg. pp. 1–41. [1]doi:10.1007/7355_2015_98.
- ↑ 23.0 23.1 Yasunori Takayama, Daisuke Uta, Hidemasa Furue, and Makoto Tominaga. Pain-enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc Natl Acad Sci 2015; 21; 112(16): 5213-5218. doi: 10.1073/pnas.1421507112. Epub 2015 Apr 6.
- ↑ Z Yilmaz, T Renton, Y Yiangou, J Zakrzewska, I P Chessell, C Bountra, P Anand. Burning mouth syndrome as a trigeminal small fibre neuropathy: Increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci. 2007 Sep;14(9):864-71. doi: 10.1016/j.jocn.2006.09.002. Epub 2007 Jun 19.