Transkranielle Magnetstimulation und Gehirnplastizität bei der Erholung nach Schlaganfall
Transkranielle Magnetstimulation und Gehirnplastizität bei der Erholung nach Schlaganfall
Abstract: This article explores the application and mechanisms of Transcranial Magnetic Stimulation (TMS) in studying neuroplasticity, particularly in the context of motor cortex reorganization after stroke. TMS, a non-invasive technique that uses magnetic fields to stimulate specific brain regions, allows researchers to map motor cortical areas and assess neuronal excitability. The study emphasizes that motor representations in the cortex are distributed over a network of primary and secondary motor areas, with overlapping motor maps that govern various muscle groups.
Research on neuroplasticity, particularly after stroke, reveals that the brain undergoes continuous reorganization, challenging the long-held belief that the adult brain is static. Following damage, areas adjacent to the injury often compensate for lost function, a phenomenon observed in both animal models and human studies. Motor and somatosensory evoked potentials, alongside magnetic stimulation, have shown to be useful in predicting functional recovery and assessing the degree of cortical reorganization.
The article discusses the importance of peripheral inputs for maintaining normal cortical organization and how their deprivation leads to significant reorganization of motor and sensory areas. Neuroplasticity mechanisms, such as unmasking pre-existing connections, synaptic modifications, and axonal sprouting, are explored in detail. Clinical findings indicate that motor recovery after stroke is associated with changes in cortical excitability and motor output, with notable interhemispheric asymmetry during the recovery process.
The conclusion highlights that motor recovery is not solely dependent on lesion resolution but involves dynamic cortical reorganization. The integration of neurophysiological tools like TMS with imaging techniques provides deeper insights into the mechanisms underlying recovery, underscoring the need for personalized rehabilitation approaches.
Introduction
Transcranial Magnetic Stimulation (TMS) is a completely harmless technique that allows specific areas of the brain to be stimulated.
By generating magnetic fields (1.5 - 2 tesla) of very short duration (< 1 msec) inside a toroid made of copper coils, and bringing the same close to the scalp, a very short-duration current is induced in the underlying brain, with the opposite direction to the current circulating in the toroid, without activating the nociceptors of the skin, muscles, or meninges.
This results in painless excitation of the neurons beneath the toroid. TMS allows mapping the connections of the motor system and defining its excitability.
The evoked response can be derived from various muscles of the body, including those of the masticatory apparatus. In fact, stimulation of the scalp about 2 cm laterally to the central point of the scalp, identified as Cz according to the International 10-20 System of Jasper, evokes an electromyographic response recordable in the pterygoid and masseter muscles contralateral to the stimulated cortical area.
Multiple Motor Cortical Maps
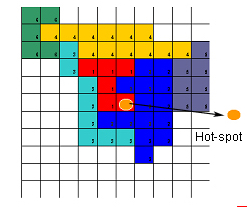
Different body parts, such as the arm, leg, or face, have predominant representation in specific brain areas, but the representations of muscles of the same body part may overlap. In this respect, the motor cortex differs from the sensory cortex, which is organized in a simpler topography. For example, the cortical motor activity maps for the muscles that move different fingers are essentially the same, while in the sensory cortex, different fingers are represented separately, like the keys of a piano. Recent studies conducted at our center using TMS, stimulating the motor cortex and recording the motor response evoked from 12 muscles of the upper limb, have demonstrated that the neuron pools that govern various muscle groups have different excitability, accompanied by different scalp representation. Some muscles, in fact, showed a tendency to organize into “clusters,” aggregating into maps that widely overlapped each other, along with well-defined and separate hot-spot zones (point from which a motor response of minimum latency and maximum amplitude can be elicited) (Rossini and Pauri 2000, Fig.2).
This observation confirms the idea that the motor cortex is distributed over a network that extends across the entire strip of the classic M1 area of Brodmann, corroborating experimental data suggesting the existence of multiple motor cortical maps from both the primary and secondary motor and premotor cortices, with multiple topographically organized descending corticospinal pathways.[1] Studies conducted with PET, fMRI, TMS, and EEG all agree on these basic concepts.
The pioneering studies by Merzenich and collaborators, conducted on monkeys, demonstrated that the organization of the sensory cortex changes after the transient or permanent loss of sensory information from a part of the body (e.g., a limb). Similar observations have been made by Donoghue and collaborators on the motor cortex. These studies showed that the cortex, no longer connected to the periphery, does not remain inactive but is “invaded” by the representation of body parts adjacent to the deafferented area, which had, in other words, been deprived of sensory information. Following reversible deafferentation, there is a temporary rearrangement of the somatotopic organization of the fingers in the primary somatosensory cortex, consisting of the expansion or displacement of cortical areas activated by the stimulation of unanesthetized fingers at the expense of cortical neurons deprived of sensory feedback.
Neuroplasticity
Continuous peripheral input from a given body region is necessary to maintain the normal somatotopic organization associated with that part of the body. [2][3][4][5][6][7][8][9]
Research on the mechanisms underlying functional recovery after stroke has led to entirely new insights into the reorganization potential of the central nervous system. Contrary to previous beliefs, the adult brain is not a static organ incapable of compensating for structural damage, but a dynamic system subject to continuous modifications.[6][10][11][12] The ability of all these flexible responses and the adaptive dynamics of the brain are defined by the term “neuroplasticity.” Thanks to rapid progress in the fields of functional imaging (PET, fMRI) and neurophysiological techniques (TMS, MEG), it is now possible to study and track over time the gradual functional recovery post-stroke. [13][14][15][16][17] Experimental observations have suggested the hypothesis of several mechanisms that may be involved in recovery: axonal sprouting, unmasking of pre-existing synaptic connections, modification of synaptic bonding in pre-existing synapses, complete synaptic reorganization. Initial studies conducted on humans have shown that similar phenomena may be responsible for adaptive processes within existing motor circuits,[18][19][20] but consistent, systematic, and anatomically correlated data on brain reorganization are still needed for more “precise system descriptions and to define theories and hypotheses”.
Intracellular recordings, MEG studies, TMS, PET, and fMRI provide information on the complex interactions of the anatomical structures involved in motor function control. Although a simple movement, such as finger flexion, can be elicited by activating a small number of pyramidal cells in the precentral gyrus, the voluntary control of such a movement is an extremely complex phenomenon involving numerous structures within the motor network, activated independently and overlapping partially over time. Movement planning involves the supplementary motor area, premotor area; [21] “executive” structures include the primary sensorimotor cortex, posterior supplementary motor area, and cingulate gyrus; [19] control structures are the cerebellum, parietal cortex, and dorsolateral prefrontal cortex (Jueptner 1998). The role of the basal ganglia is not yet entirely clear, though they seem to be involved in selecting the muscles involved in the chosen task (Jueptner 1998). One or more of these areas, or their connections, may be the site of injury, leading to reduced strength, coordination deficits, or impaired motor control. Post-lesional motor recovery cannot be solely attributed to the resolution of perilesional edema: other cellular and subcellular processes are responsible for the improvement of neurological deficits. [18] The potential for this reorganization has been largely underestimated in the past, and it is only relatively recently that we have begun to understand the organizational principles of this functional recovery.[13] [16][7] Several mechanisms are undoubtedly involved in the plasticity phenomena occurring in humans:
- Variations in the balance of excitatory and inhibitory signals can happen very quickly. This process depends on the fact that neurons or neuronal pathways extend over a broader region of anatomical connections than their usual territory of functional influence. Some areas may be held in check by tonic inhibition. If this inhibition is removed, the region of influence can rapidly expand or become “unmasked.” In studies conducted on rats, after applying bicuculline (a GABA antagonist – gamma-aminobutyric acid, a neurotransmitter with inhibitory properties) to the area of the motor cortex corresponding to the hind limb, stimulation of the nearby area, corresponding to the vibrissa, induces movements of the hind limb: this suggests that GABA-releasing neurons are crucial for maintaining proper motor cortical representation.
- Another relatively rapid mechanism involves the increase or decrease of the "strength" of pre-existing synapses, in processes such as long-term potentiation (LTP) or long-term depression (LTD). LTP and LTD occur following specific patterns of synaptic activity and can last for a long time. These processes may occur in the motor cortex.
- Changes in the excitability of the nerve cell membrane: a possible mechanism involves modifications to sodium channels in neuronal membranes, as demonstrated in conditioned variations induced in the H-reflex.
- Anatomical modifications can occur, but they require more time. Specific anatomical variations include sprouting new axon terminals and creating new synapses. For example, there may be an increase in synaptic density, which can strengthen a pre-existing, but weaker, connection. After prolonged thalamic stimulation, synaptic proliferation can be observed in the motor cortex of adult cats.
Motor and Somatosensory Evoked Potentials
Motor (MEPs) and Somatosensory (SEPs) evoked potentials, as well as evoked magnetic fields (SSEF) recorded via magnetoencephalography (MEG), can measure the integrity of the central neuronal pattern, both at the level of the descending corticospinal pathway and at the level of the ascending lemniscal and thalamocortical network. MEG provides information on variations in current flows produced in specific brain regions, in a completely non-invasive manner, with millisecond temporal resolution, and sometimes even less, allowing for tracking changes in neuronal activity that reflect signal processing. MEG is based on the assumption that current flows along the extensions of nerve cells, likened to ideal "electric cables," generate a magnetic field, which rotates around them and can be recorded with appropriate instrumentation. The resulting direction of the electric currents flowing in the dendrites is perpendicular to the layer of cortical gray matter. Compared to traditional EEG, MEG is more sensitive to tangential dipoles like those originating from the sulci rather than radial dipoles like those generated on the convexity, or other types of dipoles that generate phase opposition and thus cancel the magnetic field. MEG has enabled the representation of topographic maps of reorganization in amputees suffering from phantom limb syndrome[22] (Flor et al., 1995), demonstrating that cortical brain activity related to voluntary movements is significantly modified by induced loss of cutaneous feedback from the moving hand, that the somatotopic organization of the fingers within the sensory cortex is significantly affected by temporary anesthesia of the fingers, and that during recovery after a stroke, significant rearrangement of the hand area in S1 can be observed.[9][15][23][24]
MEPs and SSEFs have been used to assess the prognosis of motor recovery and the rehabilitative outcome, as well as reorganization in the hemisphere affected by the stroke and in the “healthy” hemisphere.[25] The integration of these two techniques has shown an important correlation with prognosis. The presence of contralateral motor responses following stimulation of the affected hemisphere is considered a good prognostic indicator. [25][26][27][28][29][30] Conversely, the absence of MEPs two weeks after the stroke correlates with poor functional recovery. The presence of ipsilateral MEPs in the unaffected hemisphere can be a bad prognostic sign,[29][31] suggesting that this form of neuroplasticity in post-stroke recovery may lead to negative functional consequences. The role of the contralateral, unaffected hemisphere in post-stroke recovery likely depends on the functions of the ipsilateral descending network to the ipsilateral muscles.[32][33]
Ipsilateral Motor Evoked Potentials
In healthy individuals, high-intensity transcranial magnetic stimulation can produce ipsilateral MEPs (iMEPs) during strong voluntary contraction.[34] [35] Since the amplitude of iMEPs can be modulated by neck rotation movements, iMEPs may be mediated by corticoreticulospinal or corticopropriospinal pathways[34] (Ziemann et al., 1999). In stroke patients, iMEPs have been elicited from the non-lesioned hemisphere[29][36][31][32] or the lesioned hemisphere[37] by stimulating anteriorly and medially relative to the primary motor cortex. The outcome of these patients is variable: some authors associate them with good recovery,[33] while others have described an association between iMEPs and poor recovery.[29] [31] It is unclear whether there are pathophysiological or methodological differences responsible for these variations. The iMEPs described by Caramia et al. [32] and Trompetto et al.[38] seem different from those described by Turton.[29] It is possible that two different groups of patients exist: in one, a large proportion of ipsilateral, non-crossed corticospinal pathways may contribute to rapid recovery. However, in most patients, there are few ipsilateral connections that may become accessible but do not fully restore lost function. Using the double-pulse paradigm, Boroojerdi and collaborators demonstrated a reduction in interhemispheric inhibition. The presence of "mirror movements," sometimes observed in stroke patients, can be attributed to the same phenomenon. Furthermore, the dorsolateral prefrontal cortex (DLPF) is likely involved in the recovery of complex movements in learning processes (Fuster 1989). Using rTMS applied to the contralateral DLPF, a significant disturbance in learning abilities has been demonstrated, while stimulation of other areas produced no effect.[39]
Cortical Reorganization Phenomena
The reorganization phenomena occurring in the sensorimotor cortices after cerebrovascular injury have been studied using an approach that integrates various investigative techniques to assess whether functional recovery is based on the re-establishment of previously damaged – but not destroyed – corticospinal connections or on the plastic rearrangement of cortical somatotopy, where functionally silent or differently operating neuron pools progressively compensate for the lost neurons. Numerous experimental findings support the hypothesis that neuronal aggregates adjacent to a lesion in the sensory and motor brain areas can progressively cover the functions previously performed by the damaged neurons. This reorganization significantly alters the interhemispheric asymmetries of the somatotopic organization of the sensorimotor cortices and implies evident clinical recovery.
An enlargement of the cortical representation of muscles regaining their function has been demonstrated following post-stroke recovery.[16] Transcranial magnetic stimulation was applied using a figure-of-eight coil to activate the motor areas responsible for controlling arm movements, particularly the hand. To construct a map, eleven positions were examined for each hemisphere, covering the precentral area. MEPs were recorded bilaterally from the ADM in awake and relaxed subjects. The TMS stimulation intensity was set at approximately 10% above the excitability threshold previously identified. For each stimulated site, four consecutive MEPs were collected. At the scalp position where MEPs of maximum amplitude and minimum latency (Hot Spot) were obtained in a relaxed state, recordings were repeated after voluntary contraction whenever patients were able to perform it. To calculate the central conduction time (CCT),[7] [8] F-waves and motor action potentials were recorded from the ADM muscles during supramaximal ulnar stimulation at the wrist.
Eighteen patients with neuroradiologically documented hemispheric stroke were studied: ten had subcortical lesions, while the other eight had cortical lesions. Recordings were performed upon admission to a rehabilitation unit, approximately 8 weeks after the stroke (T1), and during a second session, 8-10 weeks after the first (T2). Clinical conditions were tested using the Barthel disability scale and the Canadian Neurological Scale, with the hand function subscore (Hand Motor Score). The following parameters were investigated in detail: excitability threshold for the hot spot, MEP amplitude,[8] extension of the cortical motor output defined as the number of sites from which magnetic stimulation activated MEP in the target muscle, MEP onset latency from the Hot Spot, MEP onset latency from scalp sites adjacent to the Hot Spot for calculating the difference between the Hot Spot and adjacent areas, CCT calculated via the F-wave, silent period (SP) duration measured as the interval between onset and EMG latencies, interhemispheric differences in all evaluated parameters.
Normative limits were set at 2.5 SD from the mean of a group of 18 healthy subjects, in which interhemispheric differences were also measured.
In patients, the excitability thresholds in the AH were significantly higher, and the MEP amplitudes were reduced, despite the stronger stimulation intensity used.
The cortical output area corresponding to the “target” muscle was significantly and asymmetrically restricted compared to normal subjects and the unaffected hemisphere. All examined parameters were markedly more altered in T1 and in patients with subcortical lesions. These same patients had a higher number of altered neurophysiological parameters. This finding is likely attributable to the fact that a subcortical lesion affects a large number of densely grouped fibers and to lower efficiency in terms of short-term plastic reorganization, perhaps due to a longer time for achieving complete retrograde degeneration. In T2, patients with subcortical lesions showed improvement in neurophysiological parameters, matching the results of patients with cortical lesions. In this latter group, abnormal hot spot sites were more frequently observed; in these cases, recovery might have been based on the activation of brain areas outside the usual boundaries of the primary motor cortex. These results may represent a neurophysiological marker of the plastic rearrangement of cortical motor output. The MEP latency from the hot spot of the affected hemisphere was often prolonged (55% of patients). Latency differences between the hot spot and adjacent areas showed a reversal of the usual pattern – with minimum latency from the hot spot and approximately 1ms longer in nearby areas – in T1, with recovery in T2. A significant enlargement of the hand motor output area from AH was found in T2 compared to T1 in 10 out of 15 patients (excluding three patients with absent MEPs in T2): this effect was generally accompanied by clinical improvement, indicated by the Hand Motor Score, Canadian Neurological Scale, and Barthel Index.
Patients in whom hand area enlargement occurred also showed improvement in hand score. The lesion level (subcortical or cortical) did not affect the final clinical outcome.[40]
Cerebral Diaschisis
Post-stroke recovery involves mechanisms that engage the hemisphere contralateral to the ischemic lesion. The term “cerebral diaschisis” implies the idea that acute neuronal damage in the ischemic area may induce a modulatory effect on the cortical excitability of the contralateral “healthy” hemisphere through transcallosal pathways. An increase in cerebral metabolism, followed by its depression, has been documented with PET and SPECT[13] during stroke, in both the acute and subacute phases. Hyperexcitability of the contralateral hemisphere to the infarcted one has been demonstrated in animal models[41] and is considered one of the most important causes of functional recovery, resulting from cerebral plastic reorganization phenomena. Such recovery would be linked to a progressive rebalancing of excitability between the two hemispheres. In a recent study (Traversa [16]et al., 1998), two types of behavior of motor evoked potential amplitude were observed during the follow-up of patients with unilateral ischemic brain injury.
In one group of patients showing poor or no recovery 4-6 months after the stroke, the amplitude of the motor response evoked from the healthy hemisphere increased, while no changes were observed in the response recorded from the affected hemisphere, thus highlighting a progressive imbalance between the two hemispheres. In the other group, however, 4-6 months after the stroke, recovery of the evoked response from the affected hemisphere was observed, with an increase in amplitude, while the amplitude of the potential evoked from the healthy hemisphere decreased simultaneously (Fig. 3): this “balancing” response between the two hemispheres was associated with a better clinical prognosis.
A significant reorganization of motor output from the lesioned hemisphere still occurs 3-4 months after the stroke. Experimental data suggest the existence of multiple motor cortical maps from both the primary and secondary motor and premotor cortices, with multiple topographically organized descending corticospinal pathways.[1] The temporal evolution and the degree of motor recovery in humans may largely depend on the extent of lesions distributed across this motor network, as different motor areas operate in parallel rather than hierarchically-sequentially. Moreover, these parallel descending pathways may be capable of functionally compensating for each other.[37] Recordings made immediately after the stroke cannot always assess the number of intact corticospinal fibers due to either the increased excitability threshold or the conduction block of the nerve impulse (partial or total) caused by perilesional edema. The time elapsed since the stroke in our study is long enough to suggest that the observed changes are due to corticospinal tract reorganization rather than recovery from perilesional edema and cortical hypoexcitability. Recovery of sensory deficits may also play a significant role.
Conclusions
The study results in stroke patients indicate that the affected hemisphere often undergoes significant remodelling of the sensory and motor somatotopy of the hand outside the usual control areas and/or an expansion of the hand representation area. The unaffected hemisphere, in turn, undergoes a reorganization process, albeit to a lesser extent. Ultimately, interhemispheric asymmetry appears to be the parameter with the highest sensitivity in describing brain reorganization following unilateral hemispheric injury. The importance of plastic phenomena in the recovery process highlights the importance of adequate physiotherapy. Taub and collaborators have advocated for the utility of resistance movement-based physiotherapy, forcing the use of hemiplegic limbs, even in chronic and apparently stabilized conditions. An expansion of the cortical map of muscles during the recovery phase has also been demonstrated in these patients.[42] Even 6 years after a stroke, cortical motor output can be modified: with a “forced-use” paradigm, patients showed both an improvement in motor skill level and an enlargement of the motor representation area, while the cortical area related to the “healthy” limb, which was kept immobilized, contracted. Post-lesional recovery, as evidenced by the progressive normalization of cortical excitability, recovery of motor response amplitude, and latency, does not seem to be influenced by the rehabilitative method used, whether those using a peripheral-proprioceptive approach or those using a central-cognitive approach.[17]
- ↑ 1.0 1.1 Strick PL. Anatomical organization of multiple motor areas in frontal lobe: implcations for recovery of function. Adv Neurol, 1988, 47:293-312
- ↑ Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys.J Comp Neurol. 1984 Apr 20;224(4):591-605.
- ↑ Merzenich MM, Recanzone GH, Jenkins WM, Grajski KA. Adaptive mechanisms in cortical networks underlying cortical contributions to learning and nondeclarative memory. Cold Spring Harb Symp Quant Biol., 1990, 55: 873-887.
- ↑ Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys.J Comp Neurol. 1984 Apr 20;224(4):591-605.
- ↑ Pons TP, Wall JT, Garraghty PE, Cusick CG, Kaas JH.. Consistent features of the representation of the hand in area 3b of macaque monkeys.Somatosens Res. 1987;4(4):309-31.
- ↑ 6.0 6.1 Donoghue JP, Suner S, Sanes JN Dynamic organization of primary motor cortex output to target muscles in adult rats. II. Rapid reorganization following motor nerve lesions.Exp Brain Res. 1990;79(3):492-503.
- ↑ 7.0 7.1 7.2 Rossi S, Pasqualetti P, Tecchio F, Sabato A, Rossini PM.. Modulation of corticospinal output to human hand muscles following deprivation of sensory feedback. Neuroimage. 1998 Aug;8(2):163-75.
- ↑ 8.0 8.1 8.2 Rossini PM, Martino G, Narici L, Pasquarelli A, Peresson M, Pizzella V, Tecchio F, Torrioli G, Romani GL.. Short-term brain 'plasticity' in humans: transient finger representation changes in sensory cortex somatotopy following ischemic anesthesia.Brain Res. 1994 Apr 11;642(1-2):169-77
- ↑ 9.0 9.1 Rossini PM, Rossi S, Tecchio F, Pasqualetti P, Finazzi-Agrò A, Sabato A. Focal brain stimulation in healthy humans: motor maps changes following partial hand sensory deprivation. Neurosci Lett, 1996, 214: 191-195
- ↑ Holzgraefe M, Wolff JR.. A method for common undercutting of interactive regions in rat cerebral cortex.J Neurosci Methods. 1986 Dec;18(4):333-8
- ↑ Merzenich MM, Recanzone GH, Jenkins WM, Grajski KA. Adaptive mechanisms in cortical networks underlying cortical contributions to learning and nondeclarative memory. Cold Spring Harb Symp Quant Biol., 1990, 55: 873-887.
- ↑ Sanes JN, Wang J, Donoghue JP..Immediate and delayed changes of rat motor cortical output representation with new forelimb configurations. Cereb Cortex. 1992 Mar-Apr;2(2):141-52.
- ↑ 13.0 13.1 13.2 Chollet F, Di Piero V, Wise RJS, Brooks DJ, Dolan RJ, Frackowiak RSJ. The functional anatomy of motor recovery afetr stroke in humans: a study with positron emission tomography. Ann Neurol, 1991, 29: 63-71
- ↑ Friston KJ, Tononi G, Reeke GN Jr, Sporns O, Edelman .Value-dependent selection in the brain: simulation in a synthetic neural model.Neuroscience. 1994 Mar;59(2):229-43
- ↑ 15.0 15.1 Rossini PM, Tecchio F, Pizzella V, Lupoi D, Cassetta E, Pasqualetti P, Romani GL, Orlacchio A. On the reorganization of sensory hand areas after mono-hemispheric lesion: a functional (MEG)/anatomical (MRI) integrative study. Brain Res., 782 1998: 153-166
- ↑ 16.0 16.1 16.2 16.3 Traversa R, Cicinelli P, Pasqualetti P, Filippi M, Rossini PM. Follow-up of interhemispheric differences of motor evoked potentials from the “affected” and “unaffected” hemisphere in human stroke. Brain Res., 1998, 803: 1-8
- ↑ 17.0 17.1 Traversa R. et al.Neurophysiological follow-up of motor cortical output in stroke patients. Clinical Neurophysiology 111 (2000) 1695-1703
- ↑ 18.0 18.1 Cao Y, D´Olhaberriague L, Vikingstad EM Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis. Stroke 1998, 29:112-22
- ↑ 19.0 19.1 Dettmers C, Stephan KM, Lemon RN, Frackowiak RSJ. Reorganization of the executive motor system after stroke. Cerebrovasc. Dis 1997, 7: 187-200
- ↑ Weiller C, Rijntjes M. Learning, plasticity, and recovery in the central nervous system. Exp Brain Res 1999, 128:134-13
- ↑ Dettmers C, Stephan KM, Lemon RN, Frackowiak RSJ. Reorganization of the executive motor system after stroke. Cerebrovasc. Dis 1997, 7: 187-200
- ↑ Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E . Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature, 1995, 375 : 482-484
- ↑ Kristeva-Feige R, Rossi S, Pizzella V, Sabato A, Tecchio F, Feige B, Romani GL, Edrich J, Rossini PM. Changes in movement-related brain activity during transient deafferentation: a neuromagnetic study.Brain Res. 1996 Apr 1;714(1-2):201-8
- ↑ Tecchio F, Rossini PM, Pizzella V, Cassetta E, Romani GL. Spatial properties and interhemispheric differences of the sensory hand cortical representation: a magnetic study. Brain Research, 1997, 767:100-108
- ↑ 25.0 25.1 Heald A, Bates D, Cartlidge NEF, French JM, Miller S. Longitudinal study of central motor conduction time following stroke: 2. Central motor conduction time measured within 72 h after a stroke as a predictor of functional outcome at 12 months. Brain,1993, 116 : 1371- 1385
- ↑ Catano A, Houa M, Caroyer JM, Ducarne H, Noel P. Magnetic transcranial stimulation in non-hemorrhagic sylvian strokes: interest of facilitation for early functional prognosis. Electroenceph Clin Neurophysiol 1995: 97 : 349-354
- ↑ Catano A, Houa M, Caroyer JM, Ducarne H, Noel P. Magnetic transcranial stimulation in acute stroke: early excitation threshold and functional prognosis. Electroencephalogr. Clin. Neurophysiol.1996, 101: 233-239
- ↑ Binkofski F, Seitz RJ, Arnold S, Classen J, Benecke R, Freund HJ. Thalamic metabolism and corticospinal tract integrity determine motor recovery in stroke. Ann Neurol 1996: 39: 460-470.
- ↑ 29.0 29.1 29.2 29.3 29.4 Turton, A., Wroe, S., Trepte, H., Fraser, C., Lemon, R.N., Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke, Electroenceph. Clin. Neurophysiol 41 (1996) 316-328
- ↑ Rapisarda G, Bastings E, Maertens de Noordhout A, Pennisi G, Delwaide PJ (1996) Can motor recovery in stroke patients be predicted by early transcranial magnetic stimulation? Stroke 27: 2191-2196
- ↑ 31.0 31.1 31.2 Netz J, Lammers T, Hömberg V (1997) Reorganization of motor output in the non-affected hemisphere after stroke. Brain 120: 1579-1586
- ↑ 32.0 32.1 32.2 Caramia MD, Iani C, Bernardi G. Cerebral plasticity after stroke as revealed by ipsilateral responses to magnetic stimulation. Neuroreport .1996:7 1756-1760
- ↑ 33.0 33.1 Caramia MD, Palmieri MG, Giacomini P, Iani C, Dally L, Silvestrini M. Ipsilateral activation of the unaffected motor cortex in patients with hemiparetic stroke. Clin Neurophysiol 2000: 111: 1990- 1996
- ↑ 34.0 34.1 Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, Cincotta M, Wassermann EM (1999) Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol 518 : 895-906
- ↑ Alagona G, Delvaux V, Gérard P, De Pasqua V, Pennisi G, Delwaide PJ, Nicoletti F, Maertens de Noordhout . Ipsilateral motor responses to focal transcranial magnetic stimulation in healthy subjects and acute-stroke patients. Stroke2001: 32: 1304-1309
- ↑ Hendricks HT, Hageman G, van Limbeek J. Prediction of recovery from upper extremity paralysis after stroke by measuring evoked potentials. Scand J Rehabil Med 1997: 29: 155-159
- ↑ 37.0 37.1 Fries W, Danek A, Scheidtmann K, Hamburger C. Motor recovery following capsular stroke: role of descending pathways from multiple motor areas. Brain, 1993, 116: 369-382
- ↑ Trompetto C, Assini A, Buccolieri A, Marchese R, Abbruzzese G (2000) Motor recovery following stroke: a transcranial magnetic stimulation study. Clin Neurophysiol 111: 1860-1867
- ↑ Pascual-Leone A, Peris M, Tormos JM, Pascual AP, Catala MD. Reorganization of human cortical motor output maps following traumatic forearm amputation. Neuroreport 1996, 7(13):2068-2070
- ↑ Cicinelli P, Traversa R, Bassi A, Scivoletto MD and Rossini PM. Interhemispheric differences of hand muscle representation in human motor cortex. Muscle Nerve, 1997, 20:535-542
- ↑ Buchkremer-Ratzmann I, August M, Hagemann G, Witte OW. Electrophysiological transcortical diaschisis after cortical photothrombosis in rat brain. Stroke, 1996; 27: 1105-1111.
- ↑ Liepert J, Miltner WHR, Bauder H, Sommer M, Dettmers C, Taub E, Weiller C. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett1998, 250, 5-8