Jaw movements analysis. Part 1: Electrognathographic Replicator
Jaw movements analysis. Part 1: Electrognathographic Replicator
Abstract: The article delves into the diagnostic tools used for Temporomandibular Disorders (TMDs), emphasizing the limitations of electrognathographic devices like the Sirognathograph. It traces the origins of this research to critical studies from the mid-1990s, such as Lund's work, which criticized the validity of certain TMD diagnostic methods. The Research Diagnostic Criteria (RDC) were developed to standardize TMD diagnosis by eliminating many instrumental diagnostic methods that lacked clinical validation.
The article highlights key problems with these instruments, such as their inability to measure rotational movements, inaccuracies due to low sampling frequencies, and inadequate engineering modeling. Specifically, the Sirognathograph fails to capture angular data, essential for accurate mandibular kinematics, as it reduces the degrees of freedom required for such measurements. Modifications to the instrument are proposed to improve its diagnostic utility, particularly by increasing the sampling frequency. However, even with modifications, these instruments are unsuitable for fully representing the complex movements involved in TMDs.
The article concludes that while these devices are inadequate for clinical diagnosis of TMDs, they may still have a role in the differential diagnosis of orofacial pain linked to neurological conditions. It suggests that future research should focus on refining diagnostic instruments to better align with clinical needs and proposes the exploration of the Condylar Hinge Axis in upcoming chapters.
Introduction
The origins of this research can be traced back to around 1995, stemming from a series of studies by Lund, Widmer, and Feine, with a particularly interesting article for the era in which it was written: 'Validity of Diagnostic and Monitoring Tests Used for Temporomandibular Disorders' [1]. The article essentially begins as follows:
Measurements using a millimeter ruler have been used to assess mandibular mobility in large population studies of patients with TMD and asymptomatic individuals.[2] In general, the reliability of the measurements has been very good. For example, studies by Dworkin et al. (1988, 1990b) showed intraclass correlation coefficients (ICC) of 0.90 or higher when trained examiners were used.[3] [4] [5] The validity of the measurements depends on the calibration of the millimeter ruler, which is typically very good.
It is further emphasized with the following assertion:
In addition to measuring the range of motion, jaw tracking devices have been proposed to record speed, the relationship between anterior and vertical displacement during opening, regularity of movement, free vertical space, closure trajectory, and chewing movements.[6] [7] [8] [9] However, the available information suggests that the validity of the measurements is poor. For example, measurement errors ranging from 1% to 66% are reported for one instrument[10] and from 9.4% to 30% for another.[11] These instruments consistently underestimate large mandibular movements, inevitably leading to false-positive diagnoses of limited mandibular movement and potential overtreatment of healthy individuals. When data is available, other tests based on movement parameters seem to perform much worse. The speed of mandibular movement in TMD patients is below the recommended cut-off, but so is that of most normal subjects.[12][13] There are no differences between symptomatic and asymptomatic groups in the anterior/posterior displacement ratio of the mandible during opening and closing.[14], but the majority of members of both groups get positive diagnoses when the recommended cut-off is applied. On the other hand, the sensitivity of a test on the subjective classification of chewing patterns is low, as a significantly higher proportion of the symptomatic population had "good" chewing compared to the asymptomatic population.[15] A diagnosis of dyskinesia is commonly assigned.[16][17], but we could not even evaluate this test because the necessary data has not been published.
To conclude with a rather harsh thought:
However, it is clear that many clinicians who purchase electrical stimulators, EMG amplifiers, and jaw tracking devices are encouraged to use them to monitor their patients.[18] This is not justified, as there is no evidence that any of the variables measured are highly correlated with the severity of TMD.
From these initial observations, through an elaborate period of clinical research, a protocol called the Research Diagnostic Criteria has been developed, which eliminated most instrumental exams in the diagnosis of Temporomandibular Disorders (TMDs), such as the mandibular kinematic replicators that we will delve into in this chapter. The primary result of this scientific policy is reported below in Table 1.
| Table 1: TMD diagnostic methodologies analyzed and eliminated from the RDC because they are not scientifically validated | ||||
| Diagnostic Tests | Cutoff | Sensitivity | Specificity | PPV |
| Jaw movements replicator | ||||
| Mandibular opening width (Dworkin et al., 1990)[2] | Males: 35 mm
Females: 30 mm |
0.21
0.21 |
0.97
0.97 |
0.58
0.55 |
| Mandibular movement speed (Cooper and Rabuzzi, 1984)[3] | 300 mm/sec | - | 0.24 | - |
| Mandibular movement speed (Feine et al., 1988)[4] | 250 mm/sec | 1.0 | 0.20 | 0.15 |
| Anterior/Posterior relationship (Feine et al., 1988)[4] | 1/2 | 0.86 | 0.30 | 0.14 |
| Masticatory cycles (Feine et al., 1988)[4] | Descriptive | 0.26 | 0.70 | 0.11 |
| Cutoff: Parameters and limits of significance that should divide sick from healthy, for each test reported Sensitivity: Ability of the specified test to identify the truly sick in a sample of healthy and sick subjects Specificity: Ability of the specified test to identify the healthy in a sample of healthy and sick subjects Positive Predictive Value (PPV): Ratio of the ability of the specified test to identify truly sick (positive) patients on the total sick population in a sample of healthy and sick subjects. | ||||
In Table 1, indeed, the contrast in clinical value between the measurement of mouth opening with a millimeter ruler (PPV for males of 0.58 and 0.55 for females) and the analysis of mandibular kinematics performed through electrognathographic instruments (PPV below 0.20) is evident.
It should be noted and remembered that Masticationpedia follows the scientific philosophy of Feyerabend, who considers the progress of science to be a fundamentally anarchic process that responds to no authority beyond its own intellectual freedom to highlight anomalies, quantify them, and simultaneously propose new paradigms. Therefore, the assertions cited will be examined in detail to understand what is true and clinically important, while simultaneously highlighting their anomalies and rational limitations.
In this chapter, therefore, we consider one of the clinical tests strongly criticized by the Research Diagnostic Criteria and intentionally eliminated from clinical diagnostics: the replication of mandibular movements with all its characteristics such as speed, linear translations, angular translations, etc. In this context, we will consider the electrognathographic instrument that employs a magnet positioned on the mandibular incisors and lateral antennas where Hall effect sensors are placed to record the displacement of the magnetic field during mandibular movements.
We begin by noting that previous studies have made attempts to represent mandibular movement in various ways[19][20], although all systems used have their limitations. The following factors must be considered:
- The six degrees of freedom required to represent the three dimensions of movement[21][22][23];
- Non-invasiveness, to minimize intraoral disturbance;
- Linearity of the output signal[24].
In this context, we will examine the Sirognathograph electrognathographic system (Siemens, Germany)[25], providing a description of its technical aspects and proposing several modifications that should have been considered before deeming the diagnostic procedure invalid.
Instrumental Insight
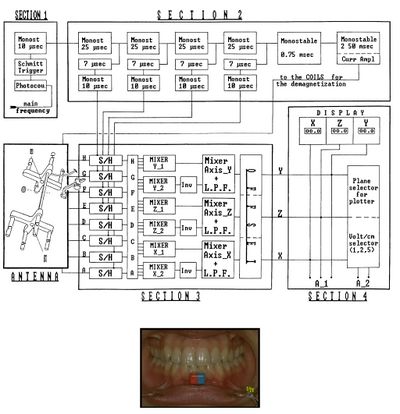
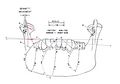
From Figure 1, we can verify the hardware structure of the instrument: Eight Hall effect sensors (marked with letters A to H) are inserted into the antenna of the Sirognathograph. They are arranged at the corners of a rectangular base parallelepiped so that the magnet remains within the solid during mandibular movements. The voltage (proportional to the magnetic field intensity) from each of the eight sensors, after appropriate mixing and filtering, provides the mandibular displacement along the three Cartesian axes: (right-left displacement), (anteroposterior displacement), and (vertical displacement). It should be noted that the sensors do not operate continuously, but are activated every 20 ms (sampling frequency 50 Hz). This imposes limits on the maximum displacement speed that can be detected (according to sampling theory, 25 Hz in our case). However, while this sampling frequency is sufficient to record normal chewing movements, it is inadequate when recording an impulsive action such as a slip on an interference or a meniscal click.
Technical Description
The electrical circuit description of the Sirognathograph can be simplified by dividing the circuit into five sections (Fig. 1).
Section 1 Section 1 is designed to input the network frequency (50-60 Hz) through a photocopier. It is then transformed into a square wave by a Schmitt trigger and a 10 μsec monostable circuit to obtain a 10 μsec pulse with a rising edge coinciding with that of the 50 Hz square wave.
Section 2 Section 2 has five monostable circuits connected in cascade that act as delay lines, four of 25 μsec and one of 0.75 msec. The first four 25 μsec pulses are used to sequentially sample the four sensor groups G/H, E/F, C/D, and A/B. After a further delay of 7 μsec, this pulse reaches the monostable circuit, which generates the sampling pulse. This 10 μsec pulse is used by the sample-and-hold circuits of section 3. The fifth monostable circuit in the chain (0.75 msec) is designed to produce a latency between sampling the last two sensors and generating a pulse to demagnetize the same sensors (see below). A 12 V pulse for 2.5 msec is sent at the end of each sampling cycle to the eight solenoids positioned near each sensor (see below). The device has 3 msec out of every 20 msec (50 Hz) available to perform its function. It remains at rest for the remaining 17 msec.
Section 3 Section 3 contains the eight sample-and-hold circuits that receive input signals from the antenna and hold them according to the sequence described above. The eight signals obtained, after appropriate mixing and filtering, provide displacement along the three axes. Displacement along the axis is obtained by first mixing the ABCD and EFGH signals. The two signals thus obtained are again mixed, but not before the polarity of the second has been inverted. The resulting signal is sent through a low-pass filter, which recreates the analog signal. The same procedure is used for the and axes, with the only difference being the combination of the eight sensors' signals. For the axis, the involved sensor groups are ABEF and CDGH, while for the axis, they are ACEG and BDFH (Figure 3). The three values obtained for the axes are sent to the offset circuit, so that the maximum intercuspation position coincides with the origin of the three axes (the zero value appears on the display).
Section 4 Section 4 is represented by three liquid crystal displays (LCD) that show the position on the three axes, then there is the voltage per centimeter selector (1-2-5), and finally, there is the plotter plane selector (xy = horizontal; xz = sagittal; yz = frontal).
Section 5 Section 5 is the antenna. In addition to its partly metal and partly plastic support, the antenna consists of eight sensors (Hall effect sensor mounted on ferrite made of semiconductor material lnSb), as well as solenoids located near each of them. The electrical potential generated by each Hall effect sensor, before being sent to the sample-and-hold circuit, is appropriately amplified by an operational amplifier in a differential configuration.
Technical Analysis
Section 1 As seen, this phase is tasked with extracting a very stable and precise frequency from the electrical network to be used as the sampling frequency (SF). This is extremely important concerning the informational content of the sampled signal and the possibility of faithfully reconstructing the original signal. Sampling theory (Nyquist theorem) states that the sampling frequency must be greater than or equal to twice the highest frequency component of the signal under analysis[26]. In our case, 50 Hz is an adequate SF to record normal masticatory activity, while it ceases to be so in the case of an impulsive act like the mandibular reflex or joint click. Indeed, if one wants to study the kinematic behavior of the mandible during the masseter reflex test, the displacement induced by the chin percussion will not be visualized because it is an impulsive act. This impulsive act must then be sampled at a higher frequency.
Section 2 The choice of sensor sampling rather than continuous analysis is due to the peculiarities of the Hall effect sensors used. The Hall effect sensor (chip) is positioned between two ferrite plates to increase its effective area, and a ferrite flux path concentrates the magnetic field in the sensor's active area[27][28][29]. These two characteristics improve the sensitivity of the sensors.
Like all ferromagnetic materials, ferrite retains some residual magnetism after the magnetic field it was exposed to is removed (magnetic hysteresis). Therefore, to obtain accurate linear measurements, the sensors (or rather, the ferrite part of the sensor) must be demagnetized using a reverse magnetic field.
The 25 msec delay lines allow for sequential sampling. This is also the time during which the sensors are powered, while the sampling lasts 10 μsec, and it is thanks to the 7 μsec delay line that it is concentrated in the time during which the sensors to be sampled are active (Fig. 1). However, it should be noted that the sensors are no longer powered at the time of the demagnetization discharge. The 0.75 msec delay line prevents the magnetic discharge from altering the measurement of the last two sampling sensors.
Section 3 After the values recorded by the sensors are held by the sample-and-hold circuits, these signals are used as described above to obtain the magnet's position relative to the three axes. Unfortunately, the Sirognathograph is not able to provide rotations on the three axes, which are of great clinical importance. The low-pass filter included in this section plays an important role in reconstructing the sampled signal. Its cutoff frequency is slightly less than half the sampling frequency. In this case, since the SF is 50 Hz, the cutoff frequency will be about 20 Hz. This is therefore the highest displacement frequency that can be measured by the Sirognathograph.
Discussion
The limit imposed on electromagnetic kinesigraphic devices used for gnathological and neurological purposes is due to the sampling frequency and measurement accuracy. These two aspects will be discussed separately.
Sampling Frequency Limit
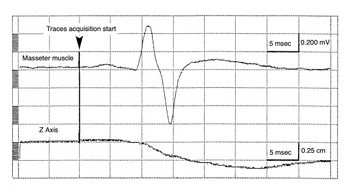
Recent studies conducted on subjects with temporomandibular dysfunction have shown that the amplitude of the mandibular reflex is reduced on the ipsilateral side of the deviation[30]. The sampling performed by the Sirognathograph is insufficient to obtain a more detailed evaluation of the relationship between the mandible's position and the mandibular reflex evoked by the chin tap with a trigger hammer. As seen in figure 2a, no deflection of the mandible downward is noted at the time of the chin tap, and in any case, the mandible begins its displacement after the onset of the muscle action potential. This is because for sampling at 50 Hz, the device acquires 1 point every 20 ms when the mandibular reflex has already ended. The chin tap can therefore be considered an impulsive act, requiring a faster sampling frequency.
A thorough analysis of the electrical circuit showed that, without making drastic changes, the SF can be increased up to a maximum of 500 Hz. Indeed, for the 20 ms period (SF = 50 Hz), the Sirognathograph completes the sampling cycle in about 3 ms and remains inactive for the remaining 17 ms. Therefore, by reducing the sampling cycle to 2 ms, the SF can be increased tenfold (500 Hz). This is possible because the latency time (0.75 ms) and saturation time (2.5 ms) have a tolerance of + 50%. Therefore, to increase the SF, some changes to the electrical circuit were necessary.
- A small board was inserted between the Schmitt trigger and the 10 μsec monostable circuit (see Fig. 1). This multiplies the network frequency by 10, producing an output frequency of 500 Hz.
- The time of the 0.75 ms monostable circuit was changed to 0.2 ms, and the 2.5 ms monostable circuit (demagnetization) to 1.25 ms.
- The cutoff frequency of the low-pass filters was changed from the original 20-25 Hz to the current 200-250 Hz.
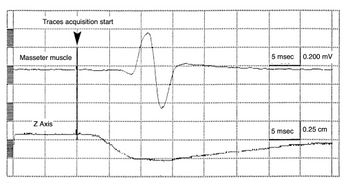
As shown in figure 2b, after the Sirognathograph was properly modified, the mandibular displacement occurs exactly 2.5 ms before the mandibular reflex. It is thus possible to perform a detailed analysis of the mandible's position, acceleration, and mechanical latency time of the displacement for subsequent gnathological studies.[31]
Limit of Angular Degrees of Freedom
As we mentioned in section 3, the eight sample-and-hold circuits that receive input signals from the antenna (Fig.1) and hold them according to the sequence described above. The eight signals obtained, after appropriate mixing and filtering, provide displacement along the three axes. Displacement along the axis is obtained by first mixing the ABCD and EFGH signals. The two signals thus obtained are again mixed, but not before the polarity of the second has been inverted. The resulting signal is sent through a low-pass filter, which recreates the analog signal. This is a crucial step that allows the colleague to understand the real physical efficiency of the diagnostic instrument. We know, in fact, that masticatory cycles are based on 6 degrees of freedom, namely 3 linear degrees of freedom and 3 angular degrees of freedom, so the long axis of the magnet, indeed, at point 8 (fig.3) does not remain parallel to the axis it had at point 1 (maximum intercuspation) but incorporates an angular rotation generated in the working condyle along the axis, a process represented in figure 4 arbitrarily quantified through modeling with Geogebra in . Due to what has been described above, the mixing of the four sensors (ABCD) with the inverted summation of the other sensors (EFGH) generates an induced electric field differential that essentially cancels the indicated angular rotation of . This has enormous repercussions on the clinical data as the speeds are differentiated between the condyles and consequently on the incisal magnet there will be an anomalous summation of linear and angular speeds precisely because of the loss of the angular data. In fact, missing the angular representation just described, the exact data of the angular speed is simultaneously missing. This means that the speed could be greater or lesser but pathognomonic of TMDs, but only due to a wider or lesser rotation on the working condyle. Obviously, this also happens for the axial plane on the axis for the same reasons, we have the loss of an angular degree of freedom by mixing, inverting, and summing the data from the CDHG and ABFE sensors.
These anomalies, therefore, are due to engineering modeling limitations that simplified the procedure by reducing the degrees of freedom to 3. This limit could have been avoided by individually sampling each Hall effect sensor, avoiding the mixing and summation on each side of the antenna. This update, however, would have required an increase in sampling frequency, which, as described, we have performed to correlate the jaw jerk to the spatial position of the mandible (Figures 2a and 2b).
This would not have been sufficient to complete the instrument and make it an actual 6-degree-of-freedom replicator because electrognathographic systems unequivocally and irremediably lose an angular degree of freedom on the axis visible on the sagittal plane (figure 5). In this case, in addition to the limit due to mixing, inversion, and summation of the Hall effect sensors (resolvable as mentioned), there would be a physical limit due to the lack of variation of the magnetic field in rotations on the axis. Practically, if a magnet is rotated between the fingers along its long axis, the magnetic field remains the same.
Mandibular kinematics, in fact, is complex, and if we consider only protrusion to facilitate the explanation, it becomes immediately evident that the condylar trace during protrusion is similar to the opening of the mandible and generates an arc at the condylar level with a center positioned in the zygomatic region (Figure 5).
The various protrusive steps generate a linear and angular displacement of the entire system with the formation of an angle (point 1-2 arbitrarily determined only to describe the concept) of 15.9°. The same phenomenon is reflected at the anterior level where the mandibular incisor moves linearly to the same extent, incorporating the counterclockwise rotation generated at the condylar level. This angular space is of fundamental importance because it constitutes the free interincisal space, an angular space necessary to allow the mandible to rotate and slide linearly during the execution of masticatory cutting activities. Indeed, the Sirognatograph and all electromagnetic gnathographic systems lose this angular data. This concept will be detailed in the specific chapters.
The various protrusive steps generate a linear and angular displacement of the entire system with the formation of an angle (point 1-2 arbitrarily determined only to describe the concept) of 15.9°. The same phenomenon is reflected at the anterior level where the mandibular incisor moves linearly to the same extent, incorporating the counterclockwise rotation generated at the condylar level. This angular space is of fundamental importance because it constitutes the free interincisal space, an angular space necessary to allow the mandible to rotate and slide linearly during the execution of masticatory cutting activities. Indeed, the Sirognatograph and all electromagnetic gnathographic systems lose this angular data. This concept will be detailed in the specific chapters.
(Certainly, but one cannot exclude instrumental diagnostic support in modern medicine because in this case, the non-correlation of clinical data is not due to errors and intellectual honesty of clinicians but due to inadequate engineering modeling of the instrument.)
Conclusions
In conclusion, since the early days of 1995 when Lund & Co. provocatively suggested eliminating a series of instrumental analyses in the diagnosis of Temporomandibular Disorders, much has changed, but the result has remained almost the same, and the variations are not yet shared by the entirety of the International Scientific Community. Let's see the most salient points:
- The RDC proposes the test of mandibular opening amplitude as a clinically valid index with a PPV of about , but we have demonstrated with clinical cases in the previous chapters that this parameter is absolutely misleading, especially when neurological pathologies with facial pain mimicking at least in the initial stages a clinical manifestation similar to TMDs are present.
- However, considering the limitations of the 'Sirognathograph' just described, there are indications for quantifying individuals with various neurological pathologies using this type of instrumentation. It has been found, for example, that Parkinson's Disease (PD) creates changes in the stomatognathic system manifesting as parafunctions that include alterations in the range of motion, pathway, and speed of mandibular movements in both PD with rigidity and tremor.[32]
- The same can be said for Dystonias, although the instrumentation has nowadays been replaced by video analysis with sensitive markers, but the concept is essentially the same, which is to analyze masticatory speed, rhythm, and direction, and of various orofacial districts.[33]
- The conceptual and methodological evolution of the RDC from 1995 to today through a massive involvement of researchers in an 'International RDC/TMD Consortium Network' focused on implementing the RDC model to expand it from TMD diagnosis to Orofacial Pain (OP) diagnosis and enable more adequate differential diagnosis has remained substantially the same. Some excerpts are reported:
- 2014-Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group† whose conclusion is as follows:
- The original diagnostic algorithms of Axis I of the RDC/TMD were reliable, but not sufficiently valid (sensitivity ≥ 0.70, specificity ≥ 0.95). For this reason, new algorithms have been developed for common pain-related TMD and an intra-articular TMJ disorder, while the Axis II tools were already valid and reliable. Two international workshops finalized the new diagnostic algorithms and Axis II tools. A group of experts modified the Axis I algorithms through workshops, using a literature review and a structured consensus process. The recommendations were assessed for validity and reliability using data from the Validation Project and the TMJ Impact Project. Axis II tools were chosen through a review of valid, short, publicly available tools already used in medical contexts. The new Axis I protocol includes a valid screening tool to detect pain-related TMD and valid diagnostic criteria to differentiate common pain-related TMD (sensitivity ≥ 0.86, specificity ≥ 0.98) and an intra-articular disorder (sensitivity 0.80, specificity 0.97). Other intra-articular disorders are suitable only for screening. Inter-examiner reliability for clinical assessment is excellent (kappa ≥ 0.85). A classification system for common and less common TMDs was presented. Axis II retains some original screening tools, supplemented with new tools to assess jaw function and behavioral and psychosocial factors. The conclusion was essentially:
The screening tools include 41 questions to assess pain intensity, pain-related disability, psychological distress, jaw functional limitations, and parafunctional behaviors, along with a pain drawing for pain sites. The comprehensive tools, with 81 questions, provide a detailed assessment of jaw functional limitations, psychological distress, anxiety, and comorbid pain conditions.
In this chapter, it has been shown how clinicians are very distant and detached from the bioengineering genesis of a diagnostic instrument they themselves use for diagnosis. We have demonstrated that the Sirognathograph along with all other similar instruments such as the Kinesiograph K7 are not three-dimensional kinematic replicators because they lose 3 degrees of freedom but above all, they are not able to identify a geometric hinge axis on which all rehabilitative gnathology is based. Therefore, in the next chapter, we will delve into this fascinating, inflated, and mysterious Condylar Hinge Axis to understand if it is clinically useful or just a source of doubt and controversy. This intermediate chapter will help us understand in detail why the RDC also eliminated the analysis of the Pantographic Replicability Index (RPI).[34]
(.....I would have eliminated electrognathography as a rehabilitative gnathological process due to engineering limitations that restrict the gnathological requirements. I would have eliminated it in the diagnosis of TMDs for the same reasons but would have kept it for differential diagnosis in Orofacial Pains that overlap with cognitive, neurodegenerative, and demyelinating neurological pathologies.)
- ↑ J P Lund, C G Widmer, J S Feine. Validity of diagnostic and monitoring tests used for temporomandibular disorders. J Dent Res. 1995 Apr;74(4):1133-43. doi: 10.1177/00220345950740041501.
- ↑ 2.0 2.1 Dworkin SF, LeResche L, DeRouen T, Von Korff MR (1990a). Assessing clinical signs of temporomandibular disorders: reliability of clinical examiners. J Prosthet Dent 63:574-579.
- ↑ 3.0 3.1 Dworkin SF, LeResche L, DeRouen T (1988). Reliability of clinical measurement in temporomandibular disorders. Clin J Pain 4:88-99.
- ↑ 4.0 4.1 4.2 4.3 Dworkin SF, LeResche L, DeRouen T, Von Korff MR (1990b). Assessing clinical signs of temporomandibular disorders: reliability of clinical examiners. J Prosthet Dent 63:574-579.
- ↑ Goulet JP, Clark GT (1990). Clinical TMJ examination methods. J CA Dent Assoc 18:25-33.
- ↑ Jankelson B, Swain CW, Crane PF, Radke JC (1975b). Kinesiometric instrumentation: a new technology. J Am Dent Assoc 90:834-840.
- ↑ Cooper BC, Rabuzzi DD (1984). Myofacial (sic) pain dysfunction syndrome: a clinical study of asymptomatic subjects. Laryngoscope 94:68-75.
- ↑ Cooper BC, Alleva M, Cooper DL, Lucente FE (1986). Myofacial (sic) pain dysfunction: an analysis of 476 patients. Laryngoscope 96:1099-1106.
- ↑ Jankelson RR (1990). Neuromuscular dental diagnosis and treatment. St. Louis: Ishiyaku EuroAmerica, Inc.
- ↑ Balkhi KM, Tallents RH (1991). Error analysis of a magnetic jaw-tracking device. J Craniomandib Disord Facial Oral Pain 5:51-56.
- ↑ Tsolka P, Woelfel JB, Man WK, Preiskel HW (1992). A laboratory assessment of recording reliability and analysis of the K6 diagnostic system. J Craniomandib Disord Facial Oral Pain 6:273-280.
- ↑ Cooper BC, Rabuzzi DD (1984). Myofacial (sic) pain dysfunction syndrome: a clinical study of asymptomatic subjects. Laryngoscope 94:68-75.
- ↑ Feine JS, Hutchins MO, Lund JP (1988). An evaluation of the criteria used to diagnose mandibular dysfunction with the mandibular kinesiograph. J Prosthet Dent 60:374-380.
- ↑ Feine JS, Hutchins MO, Lund JP (1988). An evaluation of the criteria used to diagnose mandibular dysfunction with the mandibular kinesiograph. J Prosthet Dent 60:374-380.
- ↑ Feine JS, Hutchins MO, Lund JP (1988). An evaluation of the criteria used to diagnose mandibular dysfunction with the mandibular kinesiograph. J Prosthet Dent 60:374-380.
- ↑ Cooper BC, Rabuzzi DD (1984). Myofacial (sic) pain dysfunction syndrome: a clinical study of asymptomatic subjects. Laryngoscope 94:68-75.
- ↑ Jankelson RR (1990). Neuromuscular dental diagnosis and treatment. St. Louis: Ishiyaku EuroAmerica, Inc.
- ↑ Jankelson RR (1990). Neuromuscular dental diagnosis and treatment. St. Louis: Ishiyaku EuroAmerica, Inc.
- ↑ Bates JF, Stafford GD, Harrison A: Masticatory function. A review of the literature (I). The form of the masticatory cycle. J Oral Rehabil 1975; 2: 281.
- ↑ Hannam AG: Mastication in man, in Bryant P, Gale E, Rugh J (eds): Oral Motor Behaviours: Impact on Oral Conditions and Dental Treatment. Washington DC, National Institutes of Health, 1979.
- ↑ Gillings BRO, Graham CH, Duckmanton NA: Jaw movements in young adult men during chewing. J Prosthet Dent 1973; 29: 616.
- ↑ Goodson JM, Johansen E: Analysis of human mandibular movement, in Myers HM (ed): Monographs in Oral Science. New York, S. Karger, 1975, vol 5.
- ↑ Lemmer J, Lewin A, van Rensburg LV: The measurement of jaw movement. Part I. J Prosthet Dent 1976; 36: 211.
- ↑ Hannam AG, De Cou RE, Wood WW: The kinesiographic measurement of jaw displacement. J Prosthet Dent 1980; 44: 88-93.
- ↑ Lewin A: Electrognathographics. Chicago, Quintessence Publ Co Inc, 1981.
- ↑ Leo T, Rizzolatti G: Bioingegneria della riabilitazione. Bologna, Patron Editore, 1987.
- ↑ Magnetic Sensors: Data Book. Germany, Siemens AG, Munich, 1989.
- ↑ Cuniberti E, De Lucchi L, De Stefano B: Elettronica: dispositivi e sistemi. Turin, Petrini Editore, 1988.
- ↑ Usher MJ: Sensori e trasduttori. Milan, Tecniche Nuove, 1989.
- ↑ Cruccu G, Frisardi G, Van de Stenbergher: Side asymmetry of the jaw jerk in craniomandibular dysfunction. Arch Oral Biol 1992; 4: 257-262.
- ↑ Frisardi G: The use of transcranial stimulation in the fabrication of an occlusal splint. J Prosthet Dent (in press).
- ↑ Lucas Carvalho Aragão Albuquerque, Hilton Justino da Silva. Jaw movement in people with Parkinson's Disease. Codas. 2016 Apr;28(2):193-6. doi: 10.1590/2317-1782/20162015057.
- ↑ Diego L Guarin, Babak Taati, Alessandro Abrahao, Lorne Zinman, Yana Yunusova. Video-Based Facial Movement Analysis in the Assessment of Bulbar Amyotrophic Lateral Sclerosis: Clinical Validation. J Speech Lang Hear Res. 2022 Dec 12;65(12):4667-4678. doi: 10.1044/2022_JSLHR-22-00072. Epub 2022 Nov 11.
- ↑ James M. Shields, Joseph A. Clayton, Larry D. Sindledecker. Using pantographic tracings to detect TMJ and muscle dysfunctions. J Prosthet Dent.Volume 39, Issue 1, P80-87, January 19788