Difference between revisions of "Orofacial Pain"
| Line 20: | Line 20: | ||
As usual in the presentation of new sections of specific chapters, it is advisable to introduce recent and documented references on the subject which in this case is 'Orofacial Pain' and Temporomandibular Disorders. In this sense we can partially report a brief introduction by Martina Ferrillo et al.<ref>Martina Ferrillo, Amerigo Giudice, Nicola Marotta, Francesco Fortunato,Daniela Di Venere,Antonio Ammendolia, Pietro Fiore, and Alessandro de Sire. [https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/36293017/ Pain Management and Rehabilitation for Central Sensitization in Temporomandibular Disorders: A Comprehensive Review]. Int J Mol Sci. 2022 Oct; 23(20): 12164. Published online 2022 Oct 12. doi: 10.3390/ijms232012164. PMCID: PMC9602546. PMID: 36293017</ref> on which we will make the first conceptual reflections reported by our thoughtful Linus before proceeding to the presentation of the clinical cases. | As usual in the presentation of new sections of specific chapters, it is advisable to introduce recent and documented references on the subject which in this case is 'Orofacial Pain' and Temporomandibular Disorders. In this sense we can partially report a brief introduction by Martina Ferrillo et al.<ref>Martina Ferrillo, Amerigo Giudice, Nicola Marotta, Francesco Fortunato,Daniela Di Venere,Antonio Ammendolia, Pietro Fiore, and Alessandro de Sire. [https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/36293017/ Pain Management and Rehabilitation for Central Sensitization in Temporomandibular Disorders: A Comprehensive Review]. Int J Mol Sci. 2022 Oct; 23(20): 12164. Published online 2022 Oct 12. doi: 10.3390/ijms232012164. PMCID: PMC9602546. PMID: 36293017</ref> on which we will make the first conceptual reflections reported by our thoughtful Linus before proceeding to the presentation of the clinical cases. | ||
The author points out that orofacial and neck pain comorbidities are often associated with TMD.<ref>Plesh O., Adams S.H., Gansky S.A. [https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/21837286/ Temporomandibular joint and muscle disorder-type pain and comorbid pains in a national US sample]. J. Orofac. Pain. 2011;25:190–198.</ref> These coexisting conditions (particularly headaches, migraines, and neck pain) are not only highly associated with chronic pain-related TMDs, but also increase the risk of their development.<ref>Bender S.D. Orofacial pain and headache: A review and look at the commonalities. Curr Pain Headache Rep. 2014;18:400. doi: 10.1007/s11916-013-0400-5.</ref><ref name=":0">Botros J., Gornitsky M., Samim F., der Khatchadourian Z., Velly A.M. Back and neck pain: A comparison between acute and chronic pain-related Temporomandibular Disorders. Can. J. Pain. 2022;6:112–120. doi: 10.1080/24740527.2022.2067032. </ref><ref>Ohrbach R., Fillingim R.B., Mulkey F., Gonzalez Y., Gordon S., Gremillion H., Lim P.-F., Ribeiro-Dasilva M., Greenspan J.D., Knott C. [https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/22074750/ Clinical findings and pain symptoms as potential risk factors for chronic tmd: Descriptive data and empirically identified domains from the opera case-control study.] J. Pain. 2011;12:T27–T45. doi: 10.1016/j.jpain.2011.09.001</ref> The International Classification of Headaches (ICHD)<ref>Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders; 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658.</ref> and DC/TMD<ref name=":4">Schiffman E., Ohrbach R., Truelove E., Look J., Anderson G., Goulet J.P., List T., Svensson P., Gonzalez Y., Lobbezoo F., et al. [https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/24482784/ Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†] J. Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151</ref> consider the main characteristics of pain in headache and TMD, respectively. There are several hypotheses that attempt to explain the association between TMD and headache, including neuronal convergence, central sensitization, and inhibition of descending pain downregulatory mechanisms.<ref name=":1">Matre D., Knardahl S. [https://www.degruyter.com/document/doi/10.1016/j.sjpain.2012.04.003/html ‘Central sensitization’ in chronic neck/shoulder pain]. Scand. J. Pain. 2012;3:230–235. doi: 10.1016/j.sjpain.2012.04.003. </ref><ref name=":2">Su M., Yu S. Chronic migraine: A process of dysmodulation and sensitization. Mol. Pain. 2018;14:1744806918767697. doi: 10.1177/1744806918767697.</ref> The close relationship between TMD, headache and neck pain has recently been evaluated, not only in terms of sharing common pathogenetic mechanisms and clinical features, but also considering that one condition might influence or promote the development of another.<ref>Chaves T.C., Dach F., Florencio L.L., Carvalho G.F., Gonçalves M.C., Bigal M.E., Speciali J.G., Bevilaqua-Grossi D. Concomitant Migraine and Temporomandibular Disorders are Associated With Higher Heat Pain Hyperalgesia and Cephalic Cutaneous Allodynia. Clin. J. Pain. 2016;32:882–888. doi: 10.1097/AJP.0000000000000369.</ref><ref name=":0" /><ref>Gonçalves D.A., Camparis C.M., Speciali J.G., Franco A.L., Castanharo S.M., Bigal M.E. Temporomandibular disorders are differentially associated with headache diagnoses: A controlled study. Clin. J. Pain. 2011;27:611–615. doi: 10.1097/AJP.0b013e31820e12f5.</ref> These conditions can cause facial pain and are frequently associated with the development of craniofacial allodynia during painful exacerbation.<ref name=":3">Greenspan J.D., Slade G.D., Bair E., Dubner R., Fillingim R.B., Ohrbach R., Knott C., Diatchenko L., Liu Q., Maixner W. Pain sensitivity and autonomic factors associated with development of TMD: The OPPERA prospective cohort study. J. Pain. 2013;14:T63–T74.e746. doi: 10.1016/j.jpain.2013.06.007.</ref> Indeed, pain in both conditions has been attributed to common dysfunctions of central pain regulation mechanisms..<ref>Furquim B.D., Flamengui L.M., Conti P.C. TMD and chronic pain: A current view. Dental Press J. Orthod. 2015;20:127–133. doi: 10.1590/2176-9451.20.1.127-133.sar.</ref><ref>Bevilaqua-Grossi D., Lipton R.B., Napchan U., Grosberg B., Ashina S., Bigal M.E. Temporomandibular disorders and cutaneous allodynia are associated in individuals with migraine. Cephalalgia. 2010;30:425–432. doi: 10.1111/j.1468-2982.2009.01928.x.</ref> On the other hand, the concomitant TMD and migraine showed worse levels of cutaneous hyperalgesia and allodynia, probably due to central and peripheral nervous system sensitization and impairment of descending pain modulatory pathways.<ref>Conti P.C., Costa Y.M., Gonçalves D.A., Svensson P. Headaches and myofascial temporomandibular disorders: Overlapping entities, separate managements? J. Oral Rehabil. 2016;43:702–715. doi: 10.1111/joor.12410.</ref><ref>Furquim B.D., Flamengui L.M., Conti P.C. TMD and chronic pain: A current view. Dental Press J. Orthod. 2015;20:127–133. doi: 10.1590/2176-9451.20.1.127-133.sar.</ref> | The author points out that orofacial and neck pain comorbidities are often associated with TMD.<ref>Plesh O., Adams S.H., Gansky S.A. [https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/21837286/ Temporomandibular joint and muscle disorder-type pain and comorbid pains in a national US sample]. J. Orofac. Pain. 2011;25:190–198.</ref> These coexisting conditions (particularly headaches, migraines, and neck pain) are not only highly associated with chronic pain-related TMDs, but also increase the risk of their development.<ref>Bender S.D. Orofacial pain and headache: A review and look at the commonalities. Curr Pain Headache Rep. 2014;18:400. doi: 10.1007/s11916-013-0400-5.</ref><ref name=":0">Botros J., Gornitsky M., Samim F., der Khatchadourian Z., Velly A.M. Back and neck pain: A comparison between acute and chronic pain-related Temporomandibular Disorders. Can. J. Pain. 2022;6:112–120. doi: 10.1080/24740527.2022.2067032. </ref><ref>Ohrbach R., Fillingim R.B., Mulkey F., Gonzalez Y., Gordon S., Gremillion H., Lim P.-F., Ribeiro-Dasilva M., Greenspan J.D., Knott C. [https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/22074750/ Clinical findings and pain symptoms as potential risk factors for chronic tmd: Descriptive data and empirically identified domains from the opera case-control study.] J. Pain. 2011;12:T27–T45. doi: 10.1016/j.jpain.2011.09.001</ref> The International Classification of Headaches (ICHD)<ref>Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders; 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658.</ref> and DC/TMD<ref name=":4">Schiffman E., Ohrbach R., Truelove E., Look J., Anderson G., Goulet J.P., List T., Svensson P., Gonzalez Y., Lobbezoo F., et al. [https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/24482784/ Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†] J. Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151</ref> consider the main characteristics of pain in headache and TMD, respectively. There are several hypotheses that attempt to explain the association between TMD and headache, including neuronal convergence, central sensitization, and inhibition of descending pain downregulatory mechanisms.<ref name=":1">Matre D., Knardahl S. [https://www.degruyter.com/document/doi/10.1016/j.sjpain.2012.04.003/html ‘Central sensitization’ in chronic neck/shoulder pain]. Scand. J. Pain. 2012;3:230–235. doi: 10.1016/j.sjpain.2012.04.003. </ref><ref name=":2">Su M., Yu S. Chronic migraine: A process of dysmodulation and sensitization. Mol. Pain. 2018;14:1744806918767697. doi: 10.1177/1744806918767697.</ref> The close relationship between TMD, headache and neck pain has recently been evaluated, not only in terms of sharing common pathogenetic mechanisms and clinical features, but also considering that one condition might influence or promote the development of another.<ref>Chaves T.C., Dach F., Florencio L.L., Carvalho G.F., Gonçalves M.C., Bigal M.E., Speciali J.G., Bevilaqua-Grossi D. Concomitant Migraine and Temporomandibular Disorders are Associated With Higher Heat Pain Hyperalgesia and Cephalic Cutaneous Allodynia. Clin. J. Pain. 2016;32:882–888. doi: 10.1097/AJP.0000000000000369.</ref><ref name=":0" /><ref>Gonçalves D.A., Camparis C.M., Speciali J.G., Franco A.L., Castanharo S.M., Bigal M.E. Temporomandibular disorders are differentially associated with headache diagnoses: A controlled study. Clin. J. Pain. 2011;27:611–615. doi: 10.1097/AJP.0b013e31820e12f5.</ref> These conditions can cause facial pain and are frequently associated with the development of craniofacial allodynia during painful exacerbation.<ref name=":3">Greenspan J.D., Slade G.D., Bair E., Dubner R., Fillingim R.B., Ohrbach R., Knott C., Diatchenko L., Liu Q., Maixner W. [https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/24275224/ Pain sensitivity and autonomic factors associated with development of TMD: The OPPERA prospective cohort study]. J. Pain. 2013;14:T63–T74.e746. doi: 10.1016/j.jpain.2013.06.007.</ref> Indeed, pain in both conditions has been attributed to common dysfunctions of central pain regulation mechanisms..<ref>Furquim B.D., Flamengui L.M., Conti P.C. TMD and chronic pain: A current view. Dental Press J. Orthod. 2015;20:127–133. doi: 10.1590/2176-9451.20.1.127-133.sar.</ref><ref>Bevilaqua-Grossi D., Lipton R.B., Napchan U., Grosberg B., Ashina S., Bigal M.E. Temporomandibular disorders and cutaneous allodynia are associated in individuals with migraine. Cephalalgia. 2010;30:425–432. doi: 10.1111/j.1468-2982.2009.01928.x.</ref> On the other hand, the concomitant TMD and migraine showed worse levels of cutaneous hyperalgesia and allodynia, probably due to central and peripheral nervous system sensitization and impairment of descending pain modulatory pathways.<ref>Conti P.C., Costa Y.M., Gonçalves D.A., Svensson P. Headaches and myofascial temporomandibular disorders: Overlapping entities, separate managements? J. Oral Rehabil. 2016;43:702–715. doi: 10.1111/joor.12410.</ref><ref>Furquim B.D., Flamengui L.M., Conti P.C. TMD and chronic pain: A current view. Dental Press J. Orthod. 2015;20:127–133. doi: 10.1590/2176-9451.20.1.127-133.sar.</ref> | ||
<blockquote>[[File:Question 2.jpg|50x50px|link=https://wiki.masticationpedia.org/index.php/File:Question_2.jpg|left]]'''<math>K_{brain}</math>: The uncertainty of the measurement ''' | <blockquote>[[File:Question 2.jpg|50x50px|link=https://wiki.masticationpedia.org/index.php/File:Question_2.jpg|left]]'''<math>K_{brain}</math>: The uncertainty of the measurement ''' | ||
Revision as of 18:05, 27 June 2024
Orofacial Pain
Abstract
The text provides a comprehensive discussion on the complexities of diagnosing and managing orofacial pain and temporomandibular disorders (TMD), intertwining various scientific concepts and models to better understand these conditions. It starts with an introduction to orofacial pain, specifically focusing on pain management for temporomandibular sensitization and the common coexistence of orofacial pain with headaches and neck pain. These relationships suggest mutual influences and potentially shared pathophysiological mechanisms. A significant portion of the discussion is dedicated to the correlation between TMD and other disorders like headaches, emphasizing the need to consider a broader range of pathologies when evaluating symptoms that may initially appear related to TMD. This section highlights the complex interplay between these conditions, where each may exacerbate the other, pointing towards a holistic approach to diagnosis and treatment.
The narrative then shifts towards a more theoretical discussion on statistical methodologies used in the study of TMD, comparing Bayesian and frequentist statistics. This comparison underscores the nuances of each approach, particularly focusing on the flexibility and interpretive benefits of Bayesian statistics which may offer more nuanced insights into the relationships between symptoms and underlying disorders. Furthermore, the text delves into quantum-like modeling in biological systems. It discusses the potential of quantum models to provide a more accurate interpretation of biological data compared to classical probabilistic models. This section aims to challenge and expand the reader's understanding of how complex biological interactions, like those involved in orofacial pain and TMD, can be conceptualized and analyzed.
The conclusion reiterates the complexity of TMD and orofacial pain, stressing the importance of a critical and scientifically humble approach to diagnosing and treating these conditions. It promises the presentation of clinical cases that will illustrate the differentiation between orofacial pain from TMD and from oromandibular dystonia, providing practical insights into the application of the discussed theoretical concepts. In addition to theoretical discussions, practical aspects such as the International Classification of Headache Disorders and the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) are mentioned, providing a framework for understanding the diagnostic criteria and classification used in practice.
Overall, the document underscores the intricate relationship between various pain disorders and the importance of an integrated approach to diagnosis that considers multiple potential causes and the interconnectivity of symptoms. It also emphasizes the role of innovative statistical and modeling techniques in enhancing our understanding and management of these complex conditions. This synthesis not only informs but also challenges medical professionals to think beyond traditional diagnostic paradigms and consider new, potentially more effective ways of interpreting and treating complex pain disorders.
Introduction
As usual in the presentation of new sections of specific chapters, it is advisable to introduce recent and documented references on the subject which in this case is 'Orofacial Pain' and Temporomandibular Disorders. In this sense we can partially report a brief introduction by Martina Ferrillo et al.[1] on which we will make the first conceptual reflections reported by our thoughtful Linus before proceeding to the presentation of the clinical cases.
The author points out that orofacial and neck pain comorbidities are often associated with TMD.[2] These coexisting conditions (particularly headaches, migraines, and neck pain) are not only highly associated with chronic pain-related TMDs, but also increase the risk of their development.[3][4][5] The International Classification of Headaches (ICHD)[6] and DC/TMD[7] consider the main characteristics of pain in headache and TMD, respectively. There are several hypotheses that attempt to explain the association between TMD and headache, including neuronal convergence, central sensitization, and inhibition of descending pain downregulatory mechanisms.[8][9] The close relationship between TMD, headache and neck pain has recently been evaluated, not only in terms of sharing common pathogenetic mechanisms and clinical features, but also considering that one condition might influence or promote the development of another.[10][4][11] These conditions can cause facial pain and are frequently associated with the development of craniofacial allodynia during painful exacerbation.[12] Indeed, pain in both conditions has been attributed to common dysfunctions of central pain regulation mechanisms..[13][14] On the other hand, the concomitant TMD and migraine showed worse levels of cutaneous hyperalgesia and allodynia, probably due to central and peripheral nervous system sensitization and impairment of descending pain modulatory pathways.[15][16]
: The uncertainty of the measurement
All true and, among other things, the arguments are very engaging from an intellectual point of view, but we should take into account the series of assertions such as neuronal convergence, the inhibition of downregulation mechanisms of descending pain,[8][9] allodynia,[12] and the concept of measure that inevitably incorporates an uncertainty. We reported a very interesting study (Exploring electroencephalography with a model inspired by quantum mechanics) which demonstrated the existence of an error in the measurement of the EEG by defining a similar Heisenberg uncertainty principle called quasi-quantum model which led to a constant minimum value of uncertainty in the EEG measurement at and of . Note that the unit of is the result of sampling the EEG at 250 Hz and taking mass as the unit. This should make us reflect in interpreting the results of laboratory research because, as we will see in the presentation of subsequent clinical cases, the diagnostic error is around the corner. Enough error in the specific measurement of the neuronal district under examination to make a diagnosis of Orofacial Pain when instead there is a brain tumor that involved the same nervous district and simulates the symptoms of Orofacial Pain from Temporomandibular Disorders.
Therefore, objectivity, scientific humility and a change of mindset in the interpretation of biological phenomena are needed, a topic that we will address in the 'Extraordinary Science' section At this stage, however, it is advisable to sort out the contents by resuming the already anticipated references regarding the classification of Orofacial Pain and DTM but in a more specific way to address the clinical cases to follow. Temporomandibular disorders (TMD) are a group of musculoskeletal and neuromuscular conditions affecting the masticatory muscles, temporomandibular joint (TMJ) and other associated structures.[7] According to the diagnostic criteria for TMD (DC/TMD), as already reported, in 'Axis I', TMD could be divided into intra-articular disorders, including disc displacement, arthralgia, arthritis and osteoarthritis, and muscle disorders.[7] The latter are also referred to as “myogenic TMDs”, which can be further classified into: local myalgia, if the pain is localized on palpation; myofascial pain, if the pain spreads within the palpated muscle territory; and myofascial pain with referral, if the pain spreads beyond the border of the masticatory muscles.[7]
Machine language logic
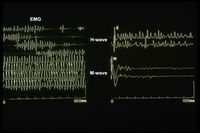
With regard to "myogenic TMDs" it is not as simple as the description of the CDR appears because, as we have highlighted for our poor patient 'Mary Poppins', the muscle pain and bone deconstruction of the ATM had concealed, in a logic of classical language, a much more serious organic damage that beyond the classifications it was possible to resolve, after 10 years of pilgrimage among various specialists. Only by acquiring a machine language logic was it possible to interpret the encrypted code of the 'Ephaptic Transmission'. (Figure 1) Having said this, classifications are welcome but not the use of a verbal language logic which remains, however, a vague phenomenon and ambiguous. Formal language logics such as mathematics are certain in the sense that equation has no solutions in the set of real numbers, because in this set there are no numbers whose square is negative. The value is then defined, called the imaginary unit, which has the following property: The equation does not exist in mathematics as it does in medical diagnostics. Without going into overly specialized topics which, however, we will address in the 'Extraordinary Science' section, in a logic of verbal language the uncertainty is much higher than that which occurs in a logic of machine language because Poppins could be affected (as they are things went) from myalgia, TMD, vasculitis, Morphea or from hemimasticatory spasm while the 'Ephaptic transmission' remains forever an organic damage and the clinical interpretation cannot be dichotomous as cannot be but only .
A recent systematic review and meta-analysis, with a combined sample of 2518 subjects, suggested that the prevalence of TMD could range from 25.2% to 34.9%,[17] with a predominance of the myofascial pain diagnosis (10.3- 15.4%) [2]. While a study by Javed Ashraf et al.[18] using Bayesian methodology, aimed to examine the association of TMD-related pain with severe headaches (migraine and TTH) over an 11-year follow-up period compared with the frequency approach. Frequentist statistics suffer from some limitations, most notably the reliance on large sample sizes to accurately determine effect sizes.[19] Furthermore, contrary to the Frequentist methodology, Bayesian statistics do not provide a (fixed) result value but rather an interval containing the regression coefficient.[20] These intervals, called credible intervals (CI), place a probability on the best estimate among all possible values of the parameter estimates.[19]
Probabilistic questions
We agree with the considerations that emerged in the study by Buchinsky et al.[18] because perhaps or fortunately we will never be able to create a formal language logic such as mathematics given the intrinsic randomness of biological models. Even the Bayes models, however, incorporate a conceptual limit which, if exceeded, would improve the probabilistic data and contextually the predictive value in the output. Briefly, Bayes' formula looks like this:
It can therefore be noted that in order to calculate the predictive value of the test, it is also necessary to know the probability with which the disease affects the overall population . Therefore, a good test is a test with sensitivity and specificity very close to 0 and we all know that this is impossible and even wrong in some ways, however, it would be a paradigmatic test. The scarce added value, in terms of information, that tumor markers, for example, provide for diagnosis, represents the rationale for which their use as a screening test in an unselected population is not recommended. The same could happen for predictive values regarding TMD culminating in a massive classification of patients and an inevitable search for the truth by the RDC project.
Without going into specialized topics, we try to briefly describe the rationale for this statement by pointing out, mainly, the differences between a classical and a quantum probabilistic model. (for more but very specialized information, see 'Quantum-like modeling in biology with open quantum systems and instruments')
Therefore, in the closed probability (CP) the probability distribution can be computed from probability and conditional probabilities . In quantum probability (QP), the classical Total Probability Formula (FTP) is perturbed by the interference term (Khrennikov, 2010);[21] for the dichotomous quantum observables and of von Neumann type, i.e. given by the Hermitian operators and , the quantum version of FTP has the form:
If the interference term is positive, then the QP computation would generate a higher probability than its CP counterpart given by the classical FTP. In particular, this probability amplification underlies the supremacy of quantum computing. There are numerous statistical data from cognitive psychology, decision making, molecular biology, genetics and epigenetics demonstrating that biosystems, from proteins and cells (Asano et al., 2015b)[22] to humans (Khrennikov, 2010,[23] Busemeyer and Bruza, 2012[24]) use this amplification and operate with non-CP updates.
If we wanted to go into a little more detail on this topic, we would immediately realize that the limit of languages lies in the fact that in medicine we are cognitively accustomed to considering the variables (symptom/disease and vice versa) dependent and therefore commutable. If a patient is symptomatic and therefore ill and a sick patient is symptomatic, this explains the terms 'dependent variables and commutability'. In quantum probability the variables are considered independent and do not commute and therefore the result could be the following:
(difficult question that needs a complex answer ... have patience and you'll see)
Conclusion
Orofacial pain together with temporomandibular disorders are very complex pathophysiological phenomena which, despite the spread of clinical protocols available to the clinician, must be considered objectively but critically. If we consider the clinical cases already presented and the limitations described regarding the measurement error, the machine language logic with the code decryption process and the limitations of the Bayesian statistical procedures we can realize as a differential diagnosis between Orofacial Pain from Temporomandibular Disorders and Orofacial Pain from Oromandibular Dystonia is not quite so appreciable. Therefore, we will present two clinical cases that will highlight the essential methodological and clinical points to perform a rapid and detailed differential diagnosis between Orofacial Pain due to Temporomandibular Disorders and Orofacial Pain due to Oromandibular Dystonia
- ↑ Martina Ferrillo, Amerigo Giudice, Nicola Marotta, Francesco Fortunato,Daniela Di Venere,Antonio Ammendolia, Pietro Fiore, and Alessandro de Sire. Pain Management and Rehabilitation for Central Sensitization in Temporomandibular Disorders: A Comprehensive Review. Int J Mol Sci. 2022 Oct; 23(20): 12164. Published online 2022 Oct 12. doi: 10.3390/ijms232012164. PMCID: PMC9602546. PMID: 36293017
- ↑ Plesh O., Adams S.H., Gansky S.A. Temporomandibular joint and muscle disorder-type pain and comorbid pains in a national US sample. J. Orofac. Pain. 2011;25:190–198.
- ↑ Bender S.D. Orofacial pain and headache: A review and look at the commonalities. Curr Pain Headache Rep. 2014;18:400. doi: 10.1007/s11916-013-0400-5.
- ↑ 4.0 4.1 Botros J., Gornitsky M., Samim F., der Khatchadourian Z., Velly A.M. Back and neck pain: A comparison between acute and chronic pain-related Temporomandibular Disorders. Can. J. Pain. 2022;6:112–120. doi: 10.1080/24740527.2022.2067032.
- ↑ Ohrbach R., Fillingim R.B., Mulkey F., Gonzalez Y., Gordon S., Gremillion H., Lim P.-F., Ribeiro-Dasilva M., Greenspan J.D., Knott C. Clinical findings and pain symptoms as potential risk factors for chronic tmd: Descriptive data and empirically identified domains from the opera case-control study. J. Pain. 2011;12:T27–T45. doi: 10.1016/j.jpain.2011.09.001
- ↑ Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders; 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658.
- ↑ 7.0 7.1 7.2 7.3 Schiffman E., Ohrbach R., Truelove E., Look J., Anderson G., Goulet J.P., List T., Svensson P., Gonzalez Y., Lobbezoo F., et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group† J. Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151
- ↑ 8.0 8.1 Matre D., Knardahl S. ‘Central sensitization’ in chronic neck/shoulder pain. Scand. J. Pain. 2012;3:230–235. doi: 10.1016/j.sjpain.2012.04.003.
- ↑ 9.0 9.1 Su M., Yu S. Chronic migraine: A process of dysmodulation and sensitization. Mol. Pain. 2018;14:1744806918767697. doi: 10.1177/1744806918767697.
- ↑ Chaves T.C., Dach F., Florencio L.L., Carvalho G.F., Gonçalves M.C., Bigal M.E., Speciali J.G., Bevilaqua-Grossi D. Concomitant Migraine and Temporomandibular Disorders are Associated With Higher Heat Pain Hyperalgesia and Cephalic Cutaneous Allodynia. Clin. J. Pain. 2016;32:882–888. doi: 10.1097/AJP.0000000000000369.
- ↑ Gonçalves D.A., Camparis C.M., Speciali J.G., Franco A.L., Castanharo S.M., Bigal M.E. Temporomandibular disorders are differentially associated with headache diagnoses: A controlled study. Clin. J. Pain. 2011;27:611–615. doi: 10.1097/AJP.0b013e31820e12f5.
- ↑ 12.0 12.1 Greenspan J.D., Slade G.D., Bair E., Dubner R., Fillingim R.B., Ohrbach R., Knott C., Diatchenko L., Liu Q., Maixner W. Pain sensitivity and autonomic factors associated with development of TMD: The OPPERA prospective cohort study. J. Pain. 2013;14:T63–T74.e746. doi: 10.1016/j.jpain.2013.06.007.
- ↑ Furquim B.D., Flamengui L.M., Conti P.C. TMD and chronic pain: A current view. Dental Press J. Orthod. 2015;20:127–133. doi: 10.1590/2176-9451.20.1.127-133.sar.
- ↑ Bevilaqua-Grossi D., Lipton R.B., Napchan U., Grosberg B., Ashina S., Bigal M.E. Temporomandibular disorders and cutaneous allodynia are associated in individuals with migraine. Cephalalgia. 2010;30:425–432. doi: 10.1111/j.1468-2982.2009.01928.x.
- ↑ Conti P.C., Costa Y.M., Gonçalves D.A., Svensson P. Headaches and myofascial temporomandibular disorders: Overlapping entities, separate managements? J. Oral Rehabil. 2016;43:702–715. doi: 10.1111/joor.12410.
- ↑ Furquim B.D., Flamengui L.M., Conti P.C. TMD and chronic pain: A current view. Dental Press J. Orthod. 2015;20:127–133. doi: 10.1590/2176-9451.20.1.127-133.sar.
- ↑ Bueno C.H., Pereira D.D., Pattussi M.P., Grossi P.K., Grossi M.L. Gender differences in temporomandibular disorders in adult populational studies: A systematic review and meta-analysis. J. Oral Rehabil. 2018;45:720–729. doi: 10.1111/joor.12661
- ↑ 18.0 18.1 Javed Ashraf,Matti Närhi, Anna Liisa Suominenand Tuomas Saxlin. Association of temporomandibular disorder-related pain with severe headaches—a Bayesian view. Clin Oral Investig. 2022; 26(1): 729–738. Published online 2021 Jul 5. doi: 10.1007/s00784-021-04051-y. PMCID: PMC8791898. PMID: 34224000
- ↑ 19.0 19.1 Buchinsky FJ, Chadha NK. To P or not to P: backing Bayesian statistics. Otolaryngol Head Neck Surg. 2017;157(6):915–918. doi: 10.1177/0194599817739260
- ↑ Depaoli S, van de Schoot R. Bayesian analyses: where to start and what to report. Eur Heal Psychol. 2014;16:75–84.
- ↑ Khrennikov A. Ubiquitous Quantum Structure: From Psychology To Finances Springer, Berlin-Heidelberg-New York(2010)
- ↑ Asano M., Khrennikov A., Ohya M., Tanaka Y., Yamato I. Quantum Adaptivity in Biology: From Genetics To Cognition Springer, Heidelberg-Berlin-New York(2015)
- ↑ Khrennikov A. Ubiquitous Quantum Structure: From Psychology To Finances Springer, Berlin-Heidelberg-New York(2010)
- ↑ Busemeyer J., Bruza P. Quantum Models of Cognition and Decision Cambridge Univ. Press, Cambridge(2012)