Difference between revisions of "5° Klinischer Fall: Spontane elektromyographische Aktivität"
(Created page with "{{main menu}} {{Versions | en = 5° Clinical case: Spontaneous Electromyographic Activity | it = 5° Caso clinico: Attività Elettromiografica Spontanea | fr = '5° Cas clinique : Activité électromyographique spontanée' | de = '5° Klinischer Fall: Spontane elektromyographische Aktivität' | es = '5° Caso Clínico: Actividad Electromiográfica Espontánea' | pt = <!-- portoghese --> | ru = <!-- russo --> | pl = <!-- polacco --> | fi = <!-- finlandese/suomi...") |
|||
| Line 16: | Line 16: | ||
[[File:EMG Propofol.jpeg|left|200x200px]] | [[File:EMG Propofol.jpeg|left|200x200px]] | ||
Wenn es um Themen wie Orofazialschmerzen (OP) oder Temporomandibuläre Störungen (TMDs) geht, stößt man oft auf Aussagen, die mehr Aufmerksamkeit verdienen, wie zum Beispiel die Aussage über den Einfluss eines einseitigen posterior Kreuzbisses auf Veränderungen der spontanen Muskelaktivität in der Ruheposition des Kiefers und bei maximaler willkürlicher Kontraktion. Diese Aussagen führen zu einem tieferen Verständnis des Phänomens der spontanen Aktivität von Motorneuronen (MUs), was angesichts der Komplexität der Faktoren und Prozesse, die bei dieser klinischen Manifestation beteiligt sind, nicht trivial ist. Aus diesem Grund präsentieren wir einen 5. klinischen Fall: Spontane elektromyographische Aktivität bei einem überwiesenen Probanden mit früherer Diagnose von TMDs. Am Ende des Kapitels werden Sie unsere Empfehlung bezüglich einer verstärkten Aufmerksamkeit für das Experimentaldesign im Bereich der trigeminalen Neurophysiologie verstehen. | |||
{{ArtBy|autore=Gianni Frisardi}} | {{ArtBy|autore=Gianni Frisardi}} | ||
{{Bookind2}} | {{Bookind2}} | ||
=== | === Einführung === | ||
ChatGPT | |||
{{q2| | In diesem Kapitel werden wir ein weiteres Thema behandeln, das viel diskutiert, aber auch viel verfolgt und als diagnostischer Test insbesondere bei Patienten mit Orofazialschmerzen (OP) und temporomandibulären Störungen (TMDs) vorgeschlagen wird: die Elektromyographie eines Muskels in Ruhezustand. Dies wirft sofort die bekannte hamletische Frage auf:{{q2|Ist ein Muskel im Ruhezustand in einem Zustand der motorischen Einheitenaktivität oder befindet er sich in einem stillen Zustand?|... vielleicht!!}} | ||
Zieliński et al.<ref>{{cita libro | Zieliński et al. <ref>{{cita libro | ||
| autore = Zieliński G | | autore = Zieliński G | ||
| autore2 = Byś A | | autore2 = Byś A | ||
| Line 46: | Line 46: | ||
| LCCN = | | LCCN = | ||
| OCLC = | | OCLC = | ||
}}</ref> | }}</ref>stellten fest, dass Veränderungen in den elektromyographischen Mustern der Kaumuskulatur mit dem Vorhandensein von Schmerzen aufgrund aktiver myofaszialer Triggerpunkte (MTrPs)<ref>{{cita libro | ||
| autore = Fernández-de-Las-Peñas C | | autore = Fernández-de-Las-Peñas C | ||
| autore2 = Galán-Del-Río F | | autore2 = Galán-Del-Río F | ||
| Line 145: | Line 145: | ||
| LCCN = | | LCCN = | ||
| OCLC = | | OCLC = | ||
}}</ref> | }}</ref> verbunden sein können und dass darüber hinaus während der elektromyographischen Untersuchung signifikant höhere Werte der Ruheaktivität innerhalb des vorderen Schläfenmuskels bei MTrPs und beobachtet wurden CMD-Patienten im Vergleich zu gesunden Personen. Die Autoren kommen zu dem Schluss, dass dieses veränderte Muster möglicherweise mit dem Vorhandensein aktiver MTrPs im Trapezmuskel zusammenhängt, die als Folge eines übertragenen Schmerzmechanismus die Aktivität des vorderen Schläfenmuskels (TA) verändern. | ||
Darüber hinaus hat derselbe Autor <ref>{{cita libro | |||
| autore = Zieliński G | | autore = Zieliński G | ||
| autore2 = Byś A | | autore2 = Byś A | ||
| Line 168: | Line 169: | ||
| LCCN = | | LCCN = | ||
| OCLC = | | OCLC = | ||
}}</ref> | }}</ref>zahlreiche klinische Studien überprüft, die zeigen, dass Depressionen erhebliche Auswirkungen auf das stomatognathe System haben, einschließlich der Aktivität der Kaumuskulatur, was zu Kiefergelenksstörungen führen kann. Darüber hinaus wurde bei Probanden mit depressiven Symptomen <ref>{{cita libro | ||
| autore = Stocka A | | autore = Stocka A | ||
| autore2 = Sierpinska T | | autore2 = Sierpinska T | ||
| Line 187: | Line 188: | ||
| LCCN = | | LCCN = | ||
| OCLC = | | OCLC = | ||
}}</ref> | }}</ref> ein Anstieg der bioelektrischen Aktivität der Kaumuskeln beobachtet. Daher ist das Ziel der Studie von Zieliński et al. Ziel war es, , quantifiziert durch das Achse-II-Protokoll von RDC/TMD, auf die bioelektrische Ruheaktivität der Kaumuskeln und Schläfenmuskeln zu bestimmen. Die Schlussfolgerung war, dass eine mittelschwere Depression, die auf der Grundlage des RDC/TMDs-II-Achsenfragebogens ermittelt wurde, nicht mit der Ruheaktivität der ausgewählten Kaumuskeln zusammenhängt und dass weitere Untersuchungen an einer größeren Gruppe von Befragten durchgeführt werden sollten, um den Zusammenhang zwischen diesen zu ermitteln psychologische Faktoren und bioelektrische Parameter der Kaumuskulatur. | ||
Unserer Meinung nach wäre es etwas komplex und vielleicht irrational, die Aktivität der Kaumuskeln im Ruhezustand bei Personen zu korrelieren, die an einer mehr oder weniger schweren Depression leiden, da das Phänomen der elektrischen Aktivität in den Ruhemuskeln im Jahr 2000 als „spontane Aktivität“ bezeichnet wird Im Fachjargon der Neurophysiologen handelt es sich um ein Phänomen mit einer nicht trivialen Erklärung. Wenn dieses Phänomen nicht zumindest weitgehend geklärt ist, kann die Vielzahl an physiopathogenetischen Interpretationen, die im Dentalbereich kursieren, zu einem diagnostischen Fehler führen. | |||
Aus diesem Grund präsentieren wir einen klinischen Fall, der über orofaziale Schmerzen (OP) und Kiefergelenksstörungen (TMDs) berichtet, bei denen in früheren medizinischen Erfahrungen leider diagnostische Schwierigkeiten aufgetreten waren. | |||
=== 5° Klinischer Fall: Spontane elektromyographische Aktivität === | |||
65-jährige weibliche Patientin berichtet hauptsächlich über Orofazialschmerzen (OP) im linken Bereich des Gesichts, insbesondere über Schmerzen, die von den Massetermuskeln zum Kiefergelenk und zum linken Schläfenmuskel ausstrahlen. Etwa 2 Jahre nach einem plötzlichen Bewusstseinsverlust, als ihr Zahnarzt einen Trochlea für die Parodontologie des unteren linken Einwanderers durchführte, begannen plötzliche Schmerzen vom unzervikalen Typ und breiteten sich dann auf das gesamte linke Hemigesicht aus, auch beim Kauen. Kollegen stellten einen Zusammenhang mit dem Kauen fest und analysierten gemäß dem RDC-Protokoll und definierten die Patientin als an temporomandibulären Störungen (TMDs) leidend. | |||
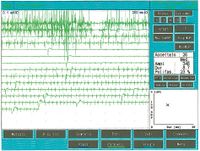
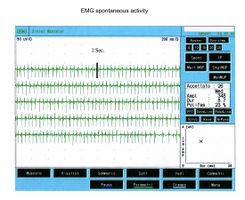
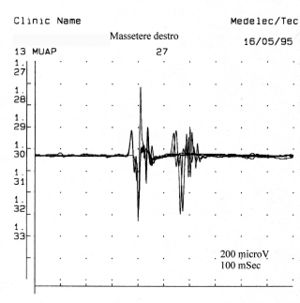
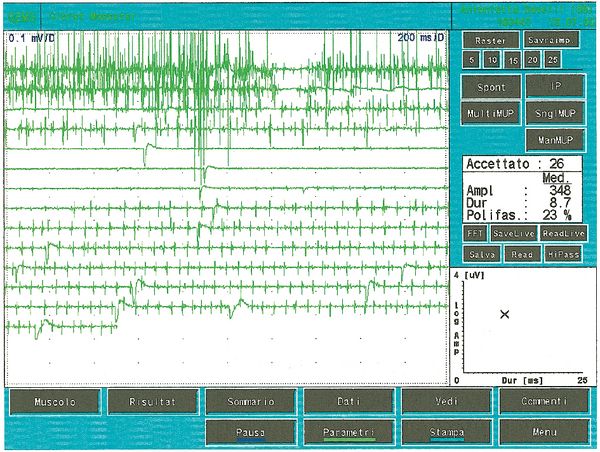
<center> | Als es uns zur Kenntnis kam, führten wir alle gnatologischen Tests durch (Axiographie, ATM-Bilder und Oberflächen-EMGs), die nicht für eine TMD sprachen, sondern für ein undefiniertes, aber im Wesentlichen neurologisches Bild. Der Grund für diese Interpretation war genau die Durchführung des Oberflächen-EMGs, das folgende Ergebnisse lieferte. Das elektromyographische Bild der Massetermuskeln wurde gemäß einer logischen Sequenz bestimmt, wie in Abbildung 1 dargestellt. Wie zu erkennen ist, war die laterale Asymmetrie der EMG-Aktivität mit Oberflächenelektroden der Massetermuskeln im entspannten Zustand (Abb. 1A), bei dem der Kiefer in Ruheposition gehalten wurde, so stark, dass eine Nadel-EMG des linken Masseters erforderlich war. Die mit dieser Technik aufgezeichnete Aktivität (Abb. 1B) zeigte eine Entladung mit einer stabilen Frequenz von 20 Hz, was eine Untersuchung der Motorneurone voraussetzte. Die Untersuchung der Motorneurone des linken Masseters (Abb. 1C) wählte automatisch 26 Motorneurone aus, deren Form, Dauer, Spitzen und Umdrehungen jedes Neurons analysiert wurden. Die Daten sind in der Tabelle (Abb. 1D) aufgeführt. Statistisch betrachtet können folgende Parameter erkannt werden: Durchschnittliche Amplitude von <math>\approxeq348\mu V</math>, eine Dauer von <math>8.7 msec</math>, <math>23%</math> polyphasischer Einheiten. Dieses klinische Bild stellt das typische pathophysiologische Phänomen dar, bei dem der Patient Schmerzen berichtet, aber die Diagnose oft "Schwierigkeiten bei der Muskelentspannung", "atypische Orofazialschmerzen" oder sogar besser "Fibromyalgie" bleibt und folglich die medikamentöse Therapie symptomatisch bleibt. Gerade diese Bedingungen sollten dem Arzt die Möglichkeit geben, seine Forschung zu vertiefen, indem er eine Koaxial-Nadel-EMG-Analyse durchführt und zumindest allgemeine Kenntnisse über deren Bestandteile hat, bevor er den Patienten an einen Neurologen überweist.<center> | ||
<gallery widths="350" heights="200" perrow="2" slideshow""=""> | <gallery widths="350" heights="200" perrow="2" slideshow""=""> | ||
File:Distonic 2.jpg|''' | File:Distonic 2.jpg|'''Abbildung 1A:''' Oberflächen-EMG zeigt eine große Aktivität der motorischen Einheiten (MUs) im linken Massetermuskel. | ||
File:Distonic 3.jpg|''' | File:Distonic 3.jpg|'''Abbildung 1B:''' Analyse der Entladungsfrequenz der Massetermotorneurone (MUs). | ||
File:Distonic 1.jpg|'''Figure 1C:''' Analysis of the morphology of the masseter motor unit (MUs) | File:Distonic 1.jpg|'''Figure 1C:''' Analysis of the morphology of the masseter motor unit (MUs) | ||
File:Distonic 4.jpg|'''Figure 1D:''' Calculation of the discharge parameters of the MUs | File:Distonic 4.jpg|'''Figure 1D:''' Calculation of the discharge parameters of the MUs | ||
</gallery> | </gallery> | ||
Revision as of 11:06, 15 March 2024
5° Klinischer Fall: Spontane elektromyographische Aktivität
Wenn es um Themen wie Orofazialschmerzen (OP) oder Temporomandibuläre Störungen (TMDs) geht, stößt man oft auf Aussagen, die mehr Aufmerksamkeit verdienen, wie zum Beispiel die Aussage über den Einfluss eines einseitigen posterior Kreuzbisses auf Veränderungen der spontanen Muskelaktivität in der Ruheposition des Kiefers und bei maximaler willkürlicher Kontraktion. Diese Aussagen führen zu einem tieferen Verständnis des Phänomens der spontanen Aktivität von Motorneuronen (MUs), was angesichts der Komplexität der Faktoren und Prozesse, die bei dieser klinischen Manifestation beteiligt sind, nicht trivial ist. Aus diesem Grund präsentieren wir einen 5. klinischen Fall: Spontane elektromyographische Aktivität bei einem überwiesenen Probanden mit früherer Diagnose von TMDs. Am Ende des Kapitels werden Sie unsere Empfehlung bezüglich einer verstärkten Aufmerksamkeit für das Experimentaldesign im Bereich der trigeminalen Neurophysiologie verstehen.
Einführung
ChatGPT
In diesem Kapitel werden wir ein weiteres Thema behandeln, das viel diskutiert, aber auch viel verfolgt und als diagnostischer Test insbesondere bei Patienten mit Orofazialschmerzen (OP) und temporomandibulären Störungen (TMDs) vorgeschlagen wird: die Elektromyographie eines Muskels in Ruhezustand. Dies wirft sofort die bekannte hamletische Frage auf:
(... vielleicht!!)
Zieliński et al. [1]stellten fest, dass Veränderungen in den elektromyographischen Mustern der Kaumuskulatur mit dem Vorhandensein von Schmerzen aufgrund aktiver myofaszialer Triggerpunkte (MTrPs)[2][3][4][5][6] verbunden sein können und dass darüber hinaus während der elektromyographischen Untersuchung signifikant höhere Werte der Ruheaktivität innerhalb des vorderen Schläfenmuskels bei MTrPs und beobachtet wurden CMD-Patienten im Vergleich zu gesunden Personen. Die Autoren kommen zu dem Schluss, dass dieses veränderte Muster möglicherweise mit dem Vorhandensein aktiver MTrPs im Trapezmuskel zusammenhängt, die als Folge eines übertragenen Schmerzmechanismus die Aktivität des vorderen Schläfenmuskels (TA) verändern.
Darüber hinaus hat derselbe Autor [7]zahlreiche klinische Studien überprüft, die zeigen, dass Depressionen erhebliche Auswirkungen auf das stomatognathe System haben, einschließlich der Aktivität der Kaumuskulatur, was zu Kiefergelenksstörungen führen kann. Darüber hinaus wurde bei Probanden mit depressiven Symptomen [8] ein Anstieg der bioelektrischen Aktivität der Kaumuskeln beobachtet. Daher ist das Ziel der Studie von Zieliński et al. Ziel war es, , quantifiziert durch das Achse-II-Protokoll von RDC/TMD, auf die bioelektrische Ruheaktivität der Kaumuskeln und Schläfenmuskeln zu bestimmen. Die Schlussfolgerung war, dass eine mittelschwere Depression, die auf der Grundlage des RDC/TMDs-II-Achsenfragebogens ermittelt wurde, nicht mit der Ruheaktivität der ausgewählten Kaumuskeln zusammenhängt und dass weitere Untersuchungen an einer größeren Gruppe von Befragten durchgeführt werden sollten, um den Zusammenhang zwischen diesen zu ermitteln psychologische Faktoren und bioelektrische Parameter der Kaumuskulatur.
Unserer Meinung nach wäre es etwas komplex und vielleicht irrational, die Aktivität der Kaumuskeln im Ruhezustand bei Personen zu korrelieren, die an einer mehr oder weniger schweren Depression leiden, da das Phänomen der elektrischen Aktivität in den Ruhemuskeln im Jahr 2000 als „spontane Aktivität“ bezeichnet wird Im Fachjargon der Neurophysiologen handelt es sich um ein Phänomen mit einer nicht trivialen Erklärung. Wenn dieses Phänomen nicht zumindest weitgehend geklärt ist, kann die Vielzahl an physiopathogenetischen Interpretationen, die im Dentalbereich kursieren, zu einem diagnostischen Fehler führen.
Aus diesem Grund präsentieren wir einen klinischen Fall, der über orofaziale Schmerzen (OP) und Kiefergelenksstörungen (TMDs) berichtet, bei denen in früheren medizinischen Erfahrungen leider diagnostische Schwierigkeiten aufgetreten waren.
5° Klinischer Fall: Spontane elektromyographische Aktivität
65-jährige weibliche Patientin berichtet hauptsächlich über Orofazialschmerzen (OP) im linken Bereich des Gesichts, insbesondere über Schmerzen, die von den Massetermuskeln zum Kiefergelenk und zum linken Schläfenmuskel ausstrahlen. Etwa 2 Jahre nach einem plötzlichen Bewusstseinsverlust, als ihr Zahnarzt einen Trochlea für die Parodontologie des unteren linken Einwanderers durchführte, begannen plötzliche Schmerzen vom unzervikalen Typ und breiteten sich dann auf das gesamte linke Hemigesicht aus, auch beim Kauen. Kollegen stellten einen Zusammenhang mit dem Kauen fest und analysierten gemäß dem RDC-Protokoll und definierten die Patientin als an temporomandibulären Störungen (TMDs) leidend.
Als es uns zur Kenntnis kam, führten wir alle gnatologischen Tests durch (Axiographie, ATM-Bilder und Oberflächen-EMGs), die nicht für eine TMD sprachen, sondern für ein undefiniertes, aber im Wesentlichen neurologisches Bild. Der Grund für diese Interpretation war genau die Durchführung des Oberflächen-EMGs, das folgende Ergebnisse lieferte. Das elektromyographische Bild der Massetermuskeln wurde gemäß einer logischen Sequenz bestimmt, wie in Abbildung 1 dargestellt. Wie zu erkennen ist, war die laterale Asymmetrie der EMG-Aktivität mit Oberflächenelektroden der Massetermuskeln im entspannten Zustand (Abb. 1A), bei dem der Kiefer in Ruheposition gehalten wurde, so stark, dass eine Nadel-EMG des linken Masseters erforderlich war. Die mit dieser Technik aufgezeichnete Aktivität (Abb. 1B) zeigte eine Entladung mit einer stabilen Frequenz von 20 Hz, was eine Untersuchung der Motorneurone voraussetzte. Die Untersuchung der Motorneurone des linken Masseters (Abb. 1C) wählte automatisch 26 Motorneurone aus, deren Form, Dauer, Spitzen und Umdrehungen jedes Neurons analysiert wurden. Die Daten sind in der Tabelle (Abb. 1D) aufgeführt. Statistisch betrachtet können folgende Parameter erkannt werden: Durchschnittliche Amplitude von , eine Dauer von , polyphasischer Einheiten. Dieses klinische Bild stellt das typische pathophysiologische Phänomen dar, bei dem der Patient Schmerzen berichtet, aber die Diagnose oft "Schwierigkeiten bei der Muskelentspannung", "atypische Orofazialschmerzen" oder sogar besser "Fibromyalgie" bleibt und folglich die medikamentöse Therapie symptomatisch bleibt. Gerade diese Bedingungen sollten dem Arzt die Möglichkeit geben, seine Forschung zu vertiefen, indem er eine Koaxial-Nadel-EMG-Analyse durchführt und zumindest allgemeine Kenntnisse über deren Bestandteile hat, bevor er den Patienten an einen Neurologen überweist.
Needle EMG steps
The EMG examination of skeletal muscles consists of four steps:
- Insertion activity when the needle electrode is inserted into the muscle
- Spontaneous activity when the muscle is at rest
- Motor unit potentials evoked by isolated motor discharges during moderate voluntary contraction
- Recruitment or interference pattern during progressive level of contraction
Insertion activity
In one subject, the insertion activity appears as high-frequency positive and negative spikes in a single group and are typically a representation of muscle fiber damage or mechanical stimulation due to needle penetration into the muscle. In our patient this activity occurred with a duration of 80 mS and was referable to a normal picture. Also note the phenomenon of plaque activity. If a needle electrode is held stationary at one point in the muscle, normal muscles at rest show absolutely no electrical activity except in the region of the neuromuscular endplate. These consist of two components: low amplitude (on the order of 10-50 μV) and low duration (1-2 msec) which to the loudspeaker EMG resemble the sound of sea shells on the ear. In our case (fig.1A) the total absence of plaque activity in the right masseter can be explained by the recording performed with surface electrodes which partially reduce the energy of the signal but the activity recorded on the left masseter, again with surface, has a width of . For the same reasoning, this activity should not be considered as plate activity since, as can be seen in fig. 1B, recording of the left masseter performed with a coaxial electrode, the amplitude is . Sometimes plaque potential spikes are indistinguishable in waveform from fibrillation potentials which also show initial negativity when recorded near the plaque. Another curious element is the similarity of the discharge model between the discharges of the neuromuscular spindles and of the plate potentials, so much so that some authors[9] hypothesized that these potentials could originate from the intrafusal muscle fibers. The discussion and the electrophysiological meaning to be given to the electrical activity observable in fig. 1B.
Spontaneous activity
In the first 2 weeks after denervation, the sensitivity of a muscle fiber to acetylcholine (ACh) increases up to 100-fold. This phenomenon known as “denervation hypersensitivity” may explain the spontaneous firing of denervated muscle fibers in response to minute ACh quanta.[10] The fact that the infusion of curare blocks the receptors of the neuromuscular plate but does not abolish the spontaneous discharge, that the denervation of the frog muscle can lead to an increased sensitivity to ACh but not generate spontaneous activity. [11]. These studies have suggested an alternative hypothesis that of slow changes in membrane potentials of metabolic origin which can periodically reach a critical level and evoke propagated spikes.[12]
These studies have suggested an alternative hypothesis that of slow changes in membrane potentials of metabolic origin which can periodically reach a critical level and evoke propagated spikes. Typical spontaneous activity phenomena, however, include fibrillation potentials, positive spike waves, fasciculation potentials, myochemical discharges, and complex repetitive discharges. Without going into overly specialized topics and considering the electrophysiological recordings of the clinical case, it is sufficient to deal with positive spike waves, fibrillation and fasciculation. Positive peak waves are sawtooth discharges that discharge spontaneously and continuously.
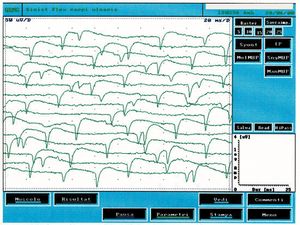
This type of activity is found in denervated muscles but also in a variety of myogenic conditions. In figure 2 it is possible to observe a typical tracing of spontaneous activity of positive peak waves which, compared with the clinical case under examination (fig.1B), are clearly different. By fibrillation, on the other hand, we mean potentials with a duration of and amplitude of with biphasic or triphasic waveforms and initial positivity. Fibrillation potentials triggered by spontaneous oscillations in the membrane potential typically fire at frequencies of with an average of . This phenomenon represents the spontaneous activity of one or more muscle fibers and is pathognomonic of denervation although it can appear in healthy muscles. The presence of reproducible discharges in at least two different areas of muscle usually suggests a secondary motor neuron disorder that includes anterior horn cell pathology, radiculopathies, plexopathies, axonal mono- and polyneuropathies as well as certain myopathies.
In figure 3 we can observe a typical trace of spontaneous activity from denervation and compare it with the trace in figure 1C in which electrophysiological differences can be noted. The spontaneous fibrillation activity has an amplitude of , the frequency appears to be random while in the reported clinical case (fig.1C) the amplitude was and the highest frequency but particularly stable almost signifying a central pacemaker.
To avoid terminological and clinical confusion between fibrillation and fasciculation Danny-Brown and Pennybacker[13] proposed the term fasciculation to describe the spontaneous twitch of motor units. Fasciculations, therefore, represent the spontaneous discharge of a group of muscle fibers referable to the entire or partial part of the motor unit. Isolated firing of a motor unit with complex bursts of repetitive firing causes vermicular movements of the skin called myokymia.[14]
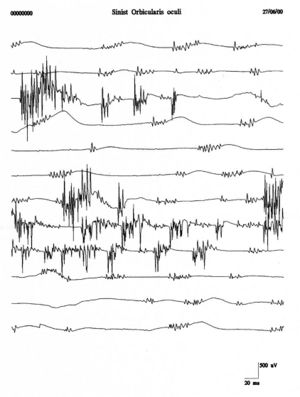
Repetitive firings of the same motor unit occur in bursts at regular intervals of with spikes firing at in each burst . Fasciculation potentials are typically associated with anterior horn cell pathologies but are also seen in radiculopathies, entrapment neuropathies, and muscle pain fasciculation syndrome. Figure 4 shows a clear example of fasciculations of the orbicularis oculi muscle which, compared with the traces of the clinical case (fig. 1B and C), shows a total morphological diversity and temporal representation. This diversity would strengthen the exclusion of a denervation pathology.
Motor Unit Potentials
A motor unit can be defined by amplitude, rise time, duration and phases as will be further described in the chapter 'Electromyography'. The recorded amplitude varies widely with the position of the electrode tip relative to the discharged ion current source, so a skilled operator selects a motor unit potential with a rise time of to be certain of proximity to the source. The amplitude in the normal range goes from hundreds to a few and the duration from . For the facial muscles, in particular, we refer to the values reported by Buchthal[15] whose range is for a maximum age of years. Biphasic or triphasic motor unit potentials are also present in normal muscles with an average of motor units with or more phases
The number of polyphasic units increases both in myopathies, neuropathies or motor neuron pathologies. Polyphasia therefore indicates a temporal dispersion of muscle fiber potentials within a motor unit. In some abnormalities called doublets or triplets a motor unit fires two or three times at a very short time interval and are representative of a metabolic disorder associated with hyperexcitability of the motoneural pool. In figure 5 we can observe a typical tracing of minimal voluntary activity of polyphasic MUAP and a double that represents a motor neuron pathology state. Comparing this recording of pathological motor unit with some of the fig.1C, and in particular the 5,7,13 and 23 with the values of respective amplitude duration and we can state that the activity recorded on the left masseter, of the clinical case in question, it has no electrophysiological characteristics that can be superimposed on a picture of damage to the II motor neuron.
Interferential pattern
Increasing the contraction greatly increases the motor units which start firing very rapidly and this precludes the identification of individual motor unit potentials. This phenomenon has been given the name of interference pattern. The density of the spikes and the average amplitude of the summed responses are determined by a series of factors such as: the descending output from the cortex, the number of motor neurons capable of firing, the firing frequency of each motor unit, the waveform of individual potentials and the probability of phase cancellation (collision). In our clinical case, the interference pattern recorded on the masseters was normal in both amplitude and frequency.
From the detailed analysis of the EMG tracing relating to the clinical case described, we can confirm the absence of organic damage to the motor unit and/or muscle fibers for the various reasons explained, such as: the absence of spontaneous activity, the normal morphology of the motor unit and interferential recruitment. The presence of EMG activity recorded on the left masseter still remains to be interpreted (fig.1) which, referring to the concepts described above, cannot be called "spontaneous activity" because it is not an expression of denervation, nor "lack of muscle relaxation" as the patient is unable to relax the muscle voluntarily or with stretching manoeuvres, nor of “EMG activity at rest due to psychic disorder as the psychometric tests are negative.
Pharmacological experimental study
An experimental study was proposed, with the consent of the patient, in which an attempt was made to pharmacologically uncouple the brainstem neuronal activity from the cortical one. Simultaneously with the pharmacological uncoupling, the EMG activity with a coaxial needle on the left masseter was monitored and contextually the blink reflex. The experimental model, which we are going to explain briefly, was created through two essential elements, namely: the choice of the specific anesthetic for the purpose of the study (propofol) and the control of the electrophysiological activity of the brainstem through the blink reflex
Propofol
The effects of anesthetics produce loss of consciousness, memory, changes in spontaneous activity, attenuation of protective reflexes, loss of postural reflexes and also adverse effects such as hallucinations, euphoria and amnesia. Furthermore they may affect the level or homeostasis of neurotransmitters in the brain such as dopamine, noraepinephrine and acetylcholine (ACh).[16] Ach was the first neurotransmitter to be described and cholinergic neurons are widely distributed in the brain. Cholinergic mechanisms are known to be important in the striatum where a balance between dopamine and ACh release ensures normal motor output,[17] hippocampus and frontal cortex where ACh plays an important role in the regulation of consciousness, memory etc.
Propofol is thought to potentiate the inhibitory effect of GABAA receptors and to have a different action from barbiturates or benzodiazepines. An elegant study[18] carried out through intracerebral microdialysis in mice demonstrated that propofol, with doses of 50 mg/kg, decreased the release of ACh from the frontal cortex by 85%, by 72% by the hippocampus and by 19% by the striatum.
Blink reflex
The blink is a reflex that is evoked by hitting the eyebrow region on one side of the forehead. Electrophysiologically it is possible to evoke it by applying an electrical stimulus on the eyebrow arch in correspondence with the supraorbital foramen. The responses are recorded through two surface electrodes positioned on the orbicularis oculi muscle on each side and the motor potentials can be mainly represented by two events, namely the ipsilateral R1 response to stimulation and the bilateral R2. These responses represent a monosynaptic and polysynaptic circuitry for R1 and R2 respectively. The R1 response was considered to follow a trigeminal pathway in the pons while the R2 via a pathway adjacent the reticular formation reaches the facial nuclei.[19][20][21]
The main neural circuitry of the blink reflex is located in the brainstem but recent work, using functional magnetic resonance imaging (fMRI), has demonstrated that two main areas in the posterior lobe of the cerebellar hemisphere, mainly on the side ipsilateral to the stimulation, are activated during the blink reflexes in humans.[22]
Experimental procedure
The experiment consisted in simultaneously monitoring the presence of the blink relex (R1 and R2) and the EMG activity of the left masseter with a needle electrode at the time of Propofol infusion at doses of which determined a slight dissociation - alert and with eyes open . In this way it can be stated, with a good approximation, that the drug released the mesencephalic-bulbar functions.
The EMG responses (fig. 6) were as follows: upon introduction of the drug there was a brief conscious loss of cortical control, which anesthetists know clinically as transient hypertonicity and which electrophysiologically causes an increase in muscle tone. In figure 6 (phase 1: first two upper traces) we can observe the increase in the discharge frequency of the motor units ranging from , before the experiment (fig.1B), to of phase 1.( Fig. 6, trace 1 and 2 above)
After the drug seems to have distributed to the cortical-subcortical areas and this effect is manifested electrophysiologically with a slowing down of the EMG discharge frequency (fig. 6 traces 3 and 4 starting from the top). After others the saturation of the cortical and, presumably, subcortical areas is complete and there is a total absence of EMG activity on the left masseter. (fig. 6 traces 5,6 and 7 starting from the top) Finally the drug is metabolised after from the electric silence and contextually the constant EMG tonic activity reappears at (fig.6 trace 8-14).
Conclusions
Experimental conclusion
The EMG activity present in the examined subject cannot be defined as "Spontaneous activity" because it does not show characteristics of organic damage to the muscle fibers and/or the second motor neuron. If it were muscle fiber damage, the EMG activity would have remained even after administration of propofol. Indeed, it was observed that doses of propofol failed to reverse the fasciculations induced by administration of 1 mg/kg of succinylcholine.[23] The EMG activity present in the subject cannot be described as "Incapacity to relax" because the term is too generic to refer to conditions of psychic disturbances and dystonic disorders. In oromandibular dystonias, in fact, there are phases of EMG silence when the patient is asked to deviate the jaw to one side in an attempt to stretch the muscle involved. This effect is determined by an additional input from the muscle proprioceptive fibers.
The disappearance of the EMG activity at doses of propofol, a dose capable of interfering with the cortical, subcortical and striatal systems while maintaining the brainstem and pontobulbar functions intact, demonstrates that the pacemaker is at a higher level of the brainstem.
The recorded EMG activity can finally be defined as "involuntary EMG activity" and responds to a pacemaker of central origin. This continuous EMG activity would determine, in the long run, damage to the myofibrils and myoglobin, an algogenic substance, would be the terminal cause of the pain reported by the patient.
Therefore, the patient was more likely to be affected by "Focal oromandibular dystonia" than by "Tempormandibular Disorders" accompanied by phenomena of bruxism even during the day. Pharmacological evidence, in fact, suggests that the central dopaminergic system may be involved in the pathogenesis of the craniocervical dystonia and bruxism.[24] Lobbezo, in fact, demonstrated by PET imaging an abnormal lateral distribution in striatal D2-binding receptors in bruxism and craniocervical dystonias.[25] The fact that peripheral trauma may cause dystonia suggests that the sensory system may be important for the pathogenesis of focal dystonia and, in any case, it can interact only if the patient is genetically predisposed to develop a post-traumatic dystonia.[26] Using PET, thermal pain stimulation of the hand and the intradermal injection of capsaicin can determine an increase in the blood flow in the contralateral putamen and globus pallidus when compared with painless thermal stimuli.[27][28] Furthermore, the expression of the genes for prodynorphin, c-Fos and c-Jun is altered at the spinal and midbrain levels after painful stimuli.[29] In dystonics, using PET, peak blood flow in response to hand vibration was significantly reduced in both primary sensory and supplementary motor cortices when compared with normal subjects.[30] Dystonic manifestations in the patients were easily elicited by the "tonic vibration reflex" but were markedly attenuated by lidocaine blockade of the muscle spindles.[31]
These authors suggested three mechanisms that may explain the increased sensitivity to vibration: the loss of the normal inhibition of the Ia afferents, a "central" alteration, and an alteration of the excitability of the neuromuscular spindles resulting from an overactivity of motor neurons. Loss of normal inhibition was also found in other experiments; in dystonics, in fact, there is a rapid curve of the recovery cycle of the blink reflex and of the H wave.[32]
Pharmacological treatment
The patient responded positively to the administration of "SIRDALUD" at doses of 4 mg three times a day more than to the administration of diazepam. Tizanidine (Sirdalud) is in fact a molecule that acts centrally as a myotonolitic agent and is pharmacologically and chemically different from diazepam and baclofen. This is a potent inhibitor of e motoneurons for stiffness experimentally induced in mice and polysynaptic activity in cats. In the decorticated or decerebrated cat, tizanidine preferentially inhibits the tonic component of the reflex activity. The actions of tizanidine result from its agonist activity at noradrenergic subunit receptors and may also involve inhibition of the release of excitatory amino acids from spinal interneurons (EAAs).[33] The action on muscle tone, the lower sedative effect compared to diazepam and baclofen and the resulting lower muscle weakness are the characteristics that led to the choice of this drug over the others (EAAs)
Clinical conclusions
To reach a clear and meaningful clinical conclusion we need to ask ourselves the following question:
Are the Temporomandibular Disorders causing a functional alteration of the trigeminal Central Nervous system or could this clinical manifestation represent a variant of a benign acute cranial polyneuritis, or a more complex clinical state?
An adequate answer to this question was given by a study by Adour KK[34] through a prospective study using neuro-otological examination and electromyography. Seven consecutive patients with cardinal symptoms of temporomandibular joint pain syndrome (pain, tenderness, clicking, and limitation of jaw movement) were evaluated within one week of the onset of their acute symptoms. Three others with chronic symptoms were tested for comparison with acute cases. All seven patients with the acute condition had asymptomatic hypoesthesia of all three divisions of the trigeminal nerve and decreased action potential of the volitional muscles in the masseter and temporal muscles. At the end of three weeks the hypesthesia resolved in all seven patients and the muscle action potential returned to normal in six of the seven. EMG testing of the single patient with persistent reduced muscle action potentials and three patients with chronic symptoms showed fibrillation, reduced polyphasic regeneration potentials, and spontaneous fasciculations with clinical atrophy and spasm of the affected masseter and temporal muscles. Other acute cranial nerve findings included unilateral glossopharyngeal and second cervical nerve hypoesthesia, motor paralysis of the superior laryngeal branch of the vagus nerve, and increased facial nerve latency. These findings suggest a neuromuscular, rather than a psychophysiological, organic cause of temporomandibular joint pain syndrome.
Contrary to this assertion that sees an organic neuromotor disturbance at the basis of a clinical situation of TMDs, there is the opinion that the influence of the unilateral posterior crossbite on the variations of spontaneous muscle activity in the mandibular rest position and in maximum voluntary contraction is significant and confirmed by Woźniak K et al.[35]
Having already clarified, albeit not in depth, the terminological, clinical and scientific difficulty in understanding phenomena that represent an alteration of the trigeminal Central Nervous System in EMG activity at rest, we can only suggest more attention in planning experiments of this type. For example Woźniak K et al.[35] reaches these conclusions by analyzing the asymmetry between sides of the EMG activity at rest and at maximum will to contract (MVC) and the algorithm used is the following:
but we did not take into account whether these asymmetries, mainly evident in the numerator, are really related to an organic symmetry of the trigeminal motor roots. Therefore, these conclusions could have become exponentially significant if correlated to the output data from the execution of the bilateral trigeminal motor Evoked Potentials bRoot-MEPs performed by our group.
This would have had an exponential clinical significance because it would have confirmed a correlation between the functional (and non-organic) asymmetry between the crossbite and the neuromotor electrical activities.
- ↑ Zieliński G, Byś A, Szkutnik J, Majcher P, Ginszt M, «Electromyographic Patterns of Masticatory Muscles in Relation to Active Myofascial Trigger Points of the Upper Trapezius and Temporomandibular Disorders», in Diagnostics (Basel), 2021».
PMID:33805008 - PMCID:PMC8063936
DOI:10.3390/diagnostics11040580
This is an Open Access resource! - ↑ Fernández-de-Las-Peñas C, Galán-Del-Río F, Alonso-Blanco C, Jiménez-García R, Arendt-Nielsen L, Svensson P, «Referred Pain from Muscle Trigger Points in the Masticatory and Neck-Shoulder Musculature in Women with Temporomandibular Disoders», in J Pain, Elsevier, 2010».
PMID:20494623
DOI:10.1016/j.jpain.2010.03.005 - ↑ Peck C, Murray G, Gerzina T, «How Does Pain Affect Jaw Muscle Activity? The Integrated Pain Adaptation Model», in Aust Dent J, 2008».
PMID:18782363
DOI:10.1111/j.1834-7819.2008.00050.x - ↑ Pietropaoli D, Ortu E, Giannoni M, Cattaneo R, Mummolo A, Monaco A, «Alterations in Surface Electromyography Are Associated with Subjective Masticatory Muscle Pain», in Pain Res Manag, 2019».
PMID:31885756 - PMCID:PMC6893259
DOI:10.1155/2019/6256179
This is an Open Access resource! - ↑ Manfredini D, Cocilovo F, Favero L, Ferronato G, Tonello S, Guarda-Nardini L, «Surface Electromyography of Jaw Muscles and Kinesiographic Recordings: Diagnostic Accuracy for Myofascial Pain», in J Oral Rehabil, Blackwell Publishing Ltd, 2011, Hoboken, New Jersey, USA».
PMID:21480942
DOI:10.1111/j.1365-2842.2011.02218.x - ↑ Simons DG, Travell JG, Simons LS, «Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual», Williams & Wilkins, 1999, Baltimore, MD, USA».
ISBN: 978-0683083637 - ↑ Zieliński G, Byś A, Ginszt M, Baszczowski JS, Majcher P, Gawda P, «Depression and Resting Masticatory Muscle Activity», in J Clin Med, 2020».
PMID:32290557 - PMCID:PMC7230290
DOI:10.3390/jcm9041097
This is an Open Access resource! - ↑ Stocka A, Sierpinska T, Kuc J, Golebiewska M, «Relationship between depression and masticatory muscles function in a group of adolescents», in Cranio, 2018».
PMID:28823222
DOI:10.1080/08869634.2017.1364030 - ↑ Partanen JV, Nousiainen U, «End-plate spikes in the electromyography are fusimotor unit potentials», in Neurology, 1983».
PMID:6683798
DOI:10.1212/wnl.33.8.1039 - ↑ Axelsson J, Thesleff S, «A study of super-sensitivity of denervated mammalian skeletal muscles», in J Physiol, 1959».
PMID:13673396 - PMCID:PMC1357014
DOI:10.1113/jphysiol.1959.sp006233 - ↑ Miledi R, «The acetylcholine sensitivity of frog musclefibres after complete or partial denervation», in J Physiol, 1960».
PMID:14422356 - PMCID:PMC1363214 - ↑ Thesleff S, «Fibrillation in denervated mammalian skeletal muscle», Oxford University Press, 1982, Oxford – in «Cukp WL e Ochoa J (eds): «Abnormal Nerves and Muscle as Impulse Genertors»».
- ↑ Danny-Brown D, Pennybacker JB.: Fibrillation and fasciculation in voluntary muscle. Brain 1938; 61: 311-332
- ↑ Sindermann F,Conrad B, Jacobi HM, Prochazka VJ.: Unusual properties of repetitive fasciculation Elctroencephalogr Clin Neurophysiol 1973; 35: 173-179
- ↑ Buchtal F: An introduction to electromyography Scandinavian University Books. Copenhagen 1957
- ↑ Angel A. : Central neuronal pathways and the process of anaesthesia. British Journal of Anaesthesia 1993; 71:148-163
- ↑ Iversen SD.: Behavioural evaluation of cholinergic drug. Life Sciences 1997; 60: 1145-1152
- ↑ Kikuchi T, Wang Y, Sato K, Okumura F.: In vivo effects of propofol on aceylcholine release from the fronatl cortex, hippocampus and striatum studied by intracerebral microdialysis in freely moving rats
- ↑ Ongerboer de Visser BW, Kuypers HG (1978): Late blink reflex changes in lateral medullary lesions. An electrophysiological and neuro-anatomical study of Wallenberg's syndrome. Brain 101: 285-294.
- ↑ Ongerboer de Visser BW (1983b): Comparative study of corneal and blink reflex latencies in patients with segmental or with cerebral lesions. In: Desmedt JE , editor. Advances in neurology. New York: Raven Press. p 757-772.
- ↑ Ongerboer de Visser BW (1983b): Comparative study of corneal and blink reflex latencies in patients with segmental or with cerebral lesions. In: Desmedt JE , editor. Advances in neurology. New York: Raven Press. p 757-772.
- ↑ Dimitrova A, Weber J, Maschke M, Elles HG, Kolb FP, Forsting M, Diener HC, Timmann D. Eyeblink-related areas in human cerebellum as shown by fMRI. Hum Brain Mapp. 2002 Oct;17(2):100-15.
- ↑ Kararmaz A. Kaya S, TurhanogluS, Ozyilmaz A.: Effects of high-dose propofol on succinylcholine-induced fasciculations and myalgia. Acta Anaesthesiol Scand 2003; 47:180-184
- ↑ Watt MW, Tan EK, Jankovic J.: Bruxism and Cranial cervical dystonia: Is there a relationship? Behavioural Sciences. 1999; 17: 196-2011)
- ↑ Lobbezzo F, Soucy JP, Montplaisir JY, Lavigne GJ.: Striatal D2 receptor binding in sleep bruxism: a controlled study with iodine 123 –iodobenzamide and single photon emission computer tomography. J Dent. Res. 1996 : 75 ; 1804-1810
- ↑ Chuldler EH, Dong WK.: The role of basal ganglia in nociception and pain Pain 1995; 60: 3-38
- ↑ Jones AK, Brown WD, Friston KJ, Qi LY,Frackowiak RS.: Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc R Soc Lond B Biol Sci 1991; 244: 39-44
- ↑ Iadarola MJ, Berman KF, Byas-Smith M, Gracely RH, Max M, Seffiro T. et all.: Positron emission tomography (PET) studies of pain and allodynia in normal and patients with chronic neuropathic pain. Soc Neurosci Abstr 1993; 19: 1074
- ↑ Bullitt E.: Introduction of c-Foslike protein whitin the lumbar spinal cord and thalamus of the rat following peripheral stimulation. Brain Res 1989; 493: 391-7
- ↑ Tempel LW, Perlmutter JS.: Abnormal cortical responses in patients with writer’s cramp Neurology 1993; 43: 2252-7
- ↑ Kaji R, Rothwell JC, Katayama M, Ikeda T, Kubori T, Kohara N, Mezaki T, Shibasaki H, Kimura J.: Tonic vibration reflex and muscle afferent block in writer's cramp. Ann Neurol. 1995 Aug;38(2):155-62
- ↑ Tolosa E, Montserrat L, Bayes A.: Blink reflex studies in focal dystonias: enhanced excitability of brainstem interneurons in cranial dystonia and spasmodic torticollis. Mov Disord. 1988;3(1):61-9
- ↑ Coward DM: The drug treatment of spasticity. Sandoz 1997
- ↑ Adour KK. Acute temporomandibular joint pain-dysfunction syndrome: neuro-otologic and electromyographic study. Am J Otolaryngol. 1981 May;2(2):114-22. doi: 10.1016/s0196-0709(81)80028-2.PMID: 7270801
- ↑ 35.0 35.1 Woźniak K, Szyszka-Sommerfeld L, Lichota D. The electrical activity of the temporal and masseter muscles in patients with TMD and unilateral posterior crossbite. Biomed Res Int. 2015;2015:259372. doi: 10.1155/2015/259372. Epub 2015 Mar 26.PMID: 25883948