Trigeminale Nozizeptive Bewertung bei TMD-Patienten durch das Studium von CO2-Laser-evozierten Potenzialen und Masseter-Laser-Stillperioden
Trigeminale Nozizeptive Bewertung bei TMD-Patienten durch das Studium von CO2-Laser-evozierten Potenzialen und Masseter-Laser-Stillperioden
Abstract: Temporomandibular dysfunction (TMD) comprises a group of painful conditions affecting the masticatory muscles and temporomandibular joint (TMJ). Despite extensive research, the pathophysiology and etiology of TMD remain unclear, with hypotheses largely unproven. Electromyographic (EMG) studies have identified alterations in the voluntary or reflex activity of the masticatory muscles in TMD patients, though these findings have not provided consistent diagnostic criteria. Recent studies refute the idea of muscle or central nervous system hyperactivity in TMD, while somatosensory system evaluations have yielded conflicting results. In this study, we aimed to investigate whether patients with unilateral chronic craniofacial pain exhibit hyposensitivity to phasic nociceptive stimuli in the trigeminal area and whether chronic pain modulates brainstem reflex circuits, specifically laser silent periods (LSP). Our results indicate that TMD patients show reduced sensitivity to phasic nociceptive stimuli, with a significant reduction in laser-evoked potential (LEP) amplitude, particularly on the painful side. Moreover, LSPs were absent in most patients, suggesting bilateral inhibition of brainstem nociceptive reflexes. These findings point to dysfunctions in the nociceptive pathways mediating and integrating phasic nociceptive inputs in TMD patients, though it remains unclear whether this dysfunction is a cause or consequence of chronic pain.
Introduction
The term temporomandibular dysfunction (TMD) refers to a group of painful syndromes affecting the masticatory muscles and the temporomandibular joint (TMJ)[1]. Nosographic classifications of TMD vary widely as they may involve different pathophysiological mechanisms, and the etiological factors are often unknown[2]. Many of the hypotheses proposed to explain its pathophysiology and etiology remain largely unproven[3][4]. Electromyographic (EMG) alterations of the voluntary or reflex activity of the masticatory muscles have often been found in association with TMD; however, EMG studies have neither provided reliable criteria nor clarified the pathogenesis of this controversial syndrome[5].
Recent studies have contradicted the hypothesis of muscle hyperactivity or a state of central nervous system hyperactivity in TMD patients[6][7][8]. Studies aimed at evaluating the somatosensory system in TMD patients have yielded conflicting results regarding the extent and detection of somatic sensitivity alterations, reporting hypersensitivity, hyposensitivity, or no alterations at all[5][9][10]. An experimental study conducted on healthy volunteers has recently shown that tonic experimental pain in the trigeminal territory reduces CO2 laser-evoked potentials (LEPs) and laser silent periods (LSPs)[11]. Since the laser stimulus selectively activates small-caliber afferents (A-delta and C fibers) and evaluates nociceptive afferents function[12][13][14], the authors concluded that tonic pain induces hypoexcitability of the trigeminal nociceptive system.
The primary aim of this study was to test the hypothesis that a well-defined group of TMD patients with unilateral chronic craniofacial pain exhibits hyposensitivity to phasic nociceptive stimuli applied in the trigeminal area. Secondly, we investigated whether brainstem reflex circuits mediating LSP are modulated by chronic craniofacial pain.
Materials and Methods
Subjects
We studied a total of 15 patients (12 women, 3 men) aged between 27 and 55 years (mean age ± SD: 39.8 ± 10.4) with a history of unilateral pain in the temporomandibular muscles or the TMJ area, and, if present, tenderness of the masticatory muscles on only one side. The inclusion criteria for TMD patients were: 1) primary diagnosis of myofascial pain and/or arthralgia according to the Research Diagnostic Criteria (RDC)[1]; 2) pain involving the masseter muscle and/or TMJ area; 3) pain duration of at least 6 months, and a weekly average pain intensity of at least 3 cm on a 0-10 cm visual analog scale (VAS). Exclusion criteria included: rheumatoid arthritis, systemic lupus erythematosus, psoriatic arthritis, fibromyalgia, and whiplash injury.
Additionally, 30 healthy control subjects comparable in age and sex to the patient group (21 women and 9 men), aged between 24 and 60 years (mean age ± SD: 43.2 ± 12.8), participated in the study. The control group subjects reported no history of craniofacial or cervical pain.
Laser-Evoked Potentials (LEPs)
A CO2 laser stimulator (Neurolas, Electronic Engineering, Florence, Italy) was used to record LEPs. Brief laser stimuli (10.6 μm; intensity 1.5-15 W; duration 10-15 ms; diameter 2.5 mm) were delivered to the perioral region (V2/V3). To avoid skin lesions and nociceptor fatigue, laser stimuli were delivered at different points within contiguous areas, with an interstimulus interval of 10-30 seconds. The perceptual threshold (PTh) was determined using the method of limits through two series of increasing and decreasing intensity stimuli. The intensity used for LEP recording was 1.5 x PTh. LEPs were recorded using surface electrodes at the vertex (Cz) with biaural references (A1, A2) and simultaneous electrooculogram recording. Two blocks of 8-12 traces each were averaged. Signals were amplified, filtered (0.5-50 Hz), and stored on a bio-potential analyzer (Premiere, Medelec, UK). For each block, the latency of N and P components and peak-to-peak amplitude were measured. Traces contaminated by ocular artifacts were excluded from the final analysis. After each block, patients were asked to report the subjective perception of the laser stimulus intensity using a 0-10 analog scale. The total duration of recorded electroencephalography activity was 1000 ms (post-stimulus).
Laser Silent Periods (LSP)
Laser silent periods in the masseter muscle were recorded using the same stimulator employed for LEPs. Electromyographic (EMG) signals were recorded bipolarly using surface electrodes applied bilaterally on the masseter muscles. Standard techniques reported in previous studies[15][16] were used for LSP recording and measurement. Twelve traces were recorded from each side in both patients and control subjects, with an interstimulus interval of 10-30 seconds. The EMG activity area for the 100 ms preceding the stimulus was measured on averaged and rectified signals. The total duration of EMG activity recorded was 100 ms pre-stimulus and 300 ms post-stimulus.
Statistical Analysis
Mean values and standard deviations are reported in the text, table, and figure. The latency of the N and P components and the peak-to-peak amplitude of LEPs were analyzed using analysis of variance (ANOVA, mixed model) between groups (control subjects and TMD patients) and within groups (between right and left sides and between painful and non-painful sides). The same statistical model was used for LSP analysis. Paired data were analyzed using Student’s t-test.
Results
Subjects
In line with the diagnostic criteria for TMD (RDC), 10 patients were classified among TMD with myofascial pain and arthralgia, and the remaining 5 were classified among TMD with craniofacial muscle myofascial pain.
Laser-Evoked Potentials (LEPs)
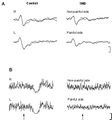
No significant differences in PTh were found between the two groups and sides (P > 0.20). However, TMD patients perceived the suprathreshold stimulus used for LEP recording as less intense on the painful side compared to the non-painful side (P < 0.05). The latency of the N and P components in patients was comparable to that of healthy controls (Figure 1).
Conversely, LEP amplitude was lower in patients compared to healthy subjects, by 30-50% (P < 0.001). Moreover, in patients, the reduction in LEP amplitude was more pronounced on the painful side (P < 0.001) (Figure 1A).
Laser Silent Periods (LSP)
In all control subjects, high-intensity laser stimuli (approximately 4 x PTh) delivered to the perioral region bilaterally elicited an EMG suppression phase during masseter muscle contraction. The latency (70.2 ± 6.8 ms) and duration (50 ± 9.8 ms) of LSP were symmetrical in control subjects. In patients, LSP was absent bilaterally in 12 of them, regardless of the stimulated side (Figure 1B). In the remaining 3 patients, 2 (TMD with myofascial pain and arthralgia) presented LSP only on the non-painful side, and 1 (TMD with myofascial pain) had normal and symmetrical LSP. Additionally, pre-stimulus EMG activity in TMD patients was reduced by 50% on the painful side and by 15% on the non-painful side compared to control subjects.
Discussion
Modulation of Laser-Evoked Potentials (LEPs)
Several studies have described modulation of brain responses evoked by phasic nociceptive experimental stimuli in patients with various types of chronic pain. In patients with the so-called "benign intractable chronic pain syndrome," a reduction in LEP amplitude compared to controls was observed[17]. LEP amplitude is also reduced in patients with cervico-brachial pain[18] and central pain[19]. A study conducted on patients with "chronic burning mouth syndrome" showed reduced sensitivity to laser stimuli both in the painful area and in non-painful areas[20]. The collective data suggest inhibition of phasic nociceptive inputs in patients with chronic pain. The results of this study on patients with chronic craniofacial pain provide further support for this interpretation.
TMD patients exhibited reduced sensitivity to phasic experimental pain, as the suprathreshold laser stimulus was perceived as less intense on the painful side compared to the non-painful side, and at the same time, LEP amplitude was significantly reduced. These observations suggest hypoactivity of the trigeminal nociceptive system projecting to the cortex in TMD patients. It is not possible to determine at which level of the central pathways this modulation occurs. However, peripheral involvement is unlikely since the perceptual threshold in TMD patients did not differ from that of controls. It is probable that the inhibition of LEP amplitude represents an alteration in the cognitive evaluation of nociceptive information, which could derive either from a psychological adaptation process, where discomfort caused by phasic experimental pain is reduced due to comparison with the considerably greater discomfort caused by clinical pain, or from a physiological phenomenon of compensatory inhibition of nociceptive information[21].
It has been hypothesized[22] that the degree of organic involvement relative to psychological involvement in different chronic pain conditions might explain the contrasting results obtained in various studies. Patients with painful paraplegia[23], neuropathic pain[24], and low back pain[25][26] exhibit an increase in the perceptual threshold for painful stimuli compared to controls. Conversely, pain conditions where psychological factors are believed to be predominant, such as fibromyalgia[27][28], or psychiatric pain[29][30], tend to be associated with a reduction in the perceptual threshold. In TMD patients, Maixner et al.[9][10] described increased sensitivity to experimental pain and suggested that pain associated with TMD may be considered a psychological disorder associated with an alteration in central inhibitory mechanisms, which consequently induces facilitation of nociceptive system activity. Conversely, a study conducted by Cruccu et al.[7] on TMD patients did not confirm a state of central hyperactivity, as TMD patients had normal excitability of the brainstem reticular formation and corticoreticular projections. It is likely that experimental differences, such as the use of different types of experimental nociceptive stimuli and the stimulation of different areas, could result in different findings in various studies.
Modulation of Laser Silent Period (LSP)
Studies on the EMG activity of muscles affected by pain have yielded conflicting results, and the literature on EMG changes in TMD provides inconclusive data[31][8]. However, there is evidence that pain alters motor performance by facilitating the motoneuron when the muscle acts as an agonist and inhibiting it when the muscle acts as an antagonist[6]. Motor performance modulation has been observed in various types of chronic pain and likely reflects an adaptive process[6]. At the trigeminal level, nociceptive inputs from the masticatory muscles reduce both the amplitude and speed of voluntary jaw movements[6] and chewing movements[32][33]. The reduction in pre-stimulus EMG activity observed in our study is consistent with the above theory. Unilateral reduction in pre-stimulus EMG activity was associated with significant bilateral inhibition of the brainstem reflex pathways mediating nociceptive reflex responses (LSPs were absent bilaterally in most TMD patients). It is unlikely that the absence of LSPs was caused by reduced pre-stimulus EMG activity. Miles and Turker[34] demonstrated that motoneuron susceptibility to inhibition is proportional to their firing rate. Therefore, at equal stimulus intensity, it should be easier to inhibit motoneurons with lower pre-stimulus firing levels[34]. Hence, the absence of LSP in TMD patients was most likely caused by chronic pain and not reduced pre-stimulus EMG activity.
LSP is a purely nociceptive reflex[35][36], whose circuit remains partially unknown. A common center has been suggested, represented by wide-dynamic-range (WDR) neurons of the reticular nucleus, corresponding to lamina V of the subcaudal nucleus[37][36], capable of mediating the second silent period of the masseter evoked electrically (SP2), the single silent period evoked by supraorbital nerve stimulation, and LSP. Experimental studies have shown that SP2 is modulated by experimental painful stimuli applied both in the trigeminal area[38] and in extra-trigeminal areas[39][40][41][42]. Conversely, data in TMD patients[43], as well as in other patients with chronic craniofacial pain[44], remain controversial.
Recently, it has been demonstrated that LSP is strongly suppressed by tonic experimental trigeminal pain, both muscular and cutaneous[11]. In this study, the absence of LSP in 12 out of 15 patients suggests a marked hypoactivity of the brainstem pathways mediating LSP. LSP was suppressed to a considerably greater extent than LEPs. It is possible that the reflex pathway is subject to dual inhibition: one mediated by a mechanism similar to that involved in LEPs (see the previous section) and another mediated by a segmental inhibition mechanism that acts on the brainstem LSP reflex circuit. Segmental inhibition can occur presynaptically, on the primary afferent fiber, or postsynaptically in the interneuronal circuit. Several studies have demonstrated presynaptic depolarization (PAD) in the trigeminal nucleus after a conditioning trigeminal stimulus[45][46]. However, while PAD may play a role in the modulation of LSP, the presynaptic inhibition mechanism alone is not sufficient to explain the bilateral suppression of LSP in our patients with unilateral pain. More likely, the pain-induced inhibitory effect occurred at the interneuronal level, along the central reflex pathway. At this level, the pain-induced effect can have a bilateral impact, as it has been demonstrated that a conditioning painful stimulus can induce contralateral segmental inhibition on dorsal horn neurons, both on nociceptive-specific neurons and wide-dynamic-range neurons[47].
In conclusion, this study demonstrated that in TMD patients with unilateral chronic pain, both nociceptive inputs directed to the cortex and the brainstem nociceptive reflex circuit are inhibited. While these results suggest a dysfunction of the nociceptive system that mediates and integrates phasic nociceptive inputs, it is not possible to assert whether this dysfunction plays a role in the pathophysiology of TMD or if it is rather a consequence of chronic pain.
- ↑ 1.0 1.1 Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations, and specifications. J Craniomandib Disord 1992; 6: 301-355.
- ↑ Green S. Case presentation: resolution of an oral lesion as a result of orofacial myofunctional therapy. Int J Orofacial Myology 2000; 26: 53-56.
- ↑ Fricton JR, Dubner R. Orofacial Pain and Temporomandibular Disorders, Advances in Pain Research and Therapy, vol. 21, Raven Press, New York; 1995.
- ↑ Sessle BJ, Bryant PS, Dionne RA. Temporomandibular Disorders and Related Pain Conditions, Progress in Pain Research and Management, vol. 4, IASP Press, Seattle, WA, 1995.
- ↑ 5.0 5.1 Svensson P, Graven-Nielsen T. Craniofacial muscle pain: review of mechanisms and clinical manifestations. J Orofacial Pain 2001; 15: 117-145.
- ↑ 6.0 6.1 6.2 6.3 Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol 1991; 69: 683-694.
- ↑ 7.0 7.1 Cruccu G, Frisardi G, Pauletti G, Romaniello A, Manfredi M. Excitability of the central masticatory pathways in patients with painful temporomandibular disorders. Pain 1997; 73: 447-454.
- ↑ 8.0 8.1 Svensson P, Arendt-Nielsen L. Clinical and experimental aspects of temporomandibular disorders. Curr Rev Pain 2000; 4: 158-165.
- ↑ 9.0 9.1 Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain 1995; 63: 341-351.
- ↑ 10.0 10.1 Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain 1998; 76: 71-81.
- ↑ 11.0 11.1 Romaniello A, Arendt-Nielsen L, Cruccu G, Svensson P. Modulation of trigeminal laser evoked potentials and laser silent periods by homotopical experimental pain. Pain, submitted.
- ↑ Bromm B, Treede RD. Laser-evoked cerebral potentials in the assessment of cutaneous pain sensitivity in normal subjects and patients. Rev Neurol (Paris) 1991; 147: 625-643.
- ↑ Cruccu G, Romaniello A, Amantini A, Lombardi M, Innocenti P, Manfredi M. Assessment of trigeminal small-fiber function: brain and reflex responses evoked by CO2-laser stimulation. Muscle Nerve 1999; 22: 508-516.
- ↑ Magerl W, Ali Z, Ellrich J, Meyer RA, Treede RD. C- and A delta-fiber components of heat-evoked cerebral potentials in healthy human subjects. Pain 1999; 82: 127-137.
- ↑ Cruccu G, Agostino R, Lahuerta J, Manfredi M. Inhibition of jaw-closing muscles by electrical stimulation of the ophthalmic division in man. Brain Res 1986; 371: 298-304.
- ↑ Ongerboer de Visser BW, Cruccu G. Neurophysiologic examination of the trigeminal, facial, hypoglossal, and spinal accessory nerves in cranial neuropathies and brain stem disorders. In: Brown WF, Bolton CF (eds). Clinical electromyography. Butterworth-Heinemann, Boston, 1993: 61-92.
- ↑ Coger RW, Kenton B, Pinsky JJ, Crue BL, Carmon A, Friedman Y. Somatosensory evoked potentials and noxious stimulation in patients with intractable, noncancer pain syndromes. Psychiatry Res 1980; 2: 279-294.
- ↑ Gibson SJ, LeVasseur SA, Helme RD. Cerebral event-related responses induced by CO2 laser stimulation in subjects suffering from cervico-brachial syndrome. Pain 1991; 47: 173-182.
- ↑ Casey KL, Beydoun A, Boivie J, Sjolund B, Holmgren H, Leijon G, Morrow TJ, Rosen I. Laser-evoked cerebral potentials and sensory function in patients with central pain. Pain 1996; 64: 485-491.
- ↑ Svensson P, Bjerring P, Arendt-Nielsen L, Kaaber S. Sensory and pain thresholds to orofacial argon laser stimulation in patients with chronic burning mouth syndrome. Clin J Pain 1993; 9:207-215.
- ↑ Gracely RH. Subjective quantification of pain perception. In: Bromm B (ed.), Pain Measurement in Man: Neurophysiological Correlates of Pain, Elsevier, Amsterdam, 1984: 111-137.
- ↑ Langermark M, Jensen K, Jensen TS, Olesen J. Pressure pain thresholds and thermal nociceptive thresholds in chronic tension-type headache. Pain 1989; 38: 203-210.
- ↑ Hazouri LA, Mueller AD. Pain threshold studies on paraplegic patients. Arch Neurol Psychiatry 1950; 64: 607-613.
- ↑ Callaghan M, Sternbach RA, Nyquist JK, Timmermans G. Changes in somatic sensitivity during transcutaneous electrical analgesia. Pain 1978; 5:115-127.
- ↑ Naliboff BD, Cohen MJ, Schandler SL, Heinrich RL. Signal detection and threshold measures for chronic low back pain patients, chronic illness, and cohort controls to radiant heat stimuli. J Abnorm Psychol 1981; 90: 271-274.
- ↑ Cohen MJ, Naliboff BD, Schandler SL, Heinrich RL. Signal detection and threshold measures to loud tones and radiant heat in chronic low back pain patients and cohort controls. Pain 1983; 16: 245-252.
- ↑ Quimby LG, Block SR, Gratwick GM. Fibromyalgia: generalized pain intolerance and manifold symptom reporting. J Rheumatol 1988; 15: 1264-1270.
- ↑ Scudd RA, Rollman GB, Harth M, McCain GA. Pain perception and personality measures as discriminators in the classification of fibrositis. J Rheumatol 1987; 14: 563-569.
- ↑ Merskey H. The effect of chronic pain upon response to noxious stimuli by psychiatric patients. J Psychosom Res 1964; 8: 405-419.
- ↑ Merskey H, Evans PR. Variations in pain complaint threshold in psychiatric and neurological patients with pain. Pain 1975; 1: 73-79.
- ↑ Stohler CS. Craniofacial Pain and Motor Function, Exp Brain Res 1995; 99: 46-53.
- ↑ Svensson P, Arendt-Nielsen L, Bjerring P, Bak P, Hjorth T, Troest T. Human mastication modulated by experimental trigeminal and extra-trigeminal painful stimuli. J Oral Rehabil 1996a; 23: 838-848.
- ↑ Svensson P, Arendt-Nielsen L, Houe L. Sensory-motor interactions of human experimental unilateral jaw muscle pain: a quantitative analysis. Pain 1996b; 64: 241-249.
- ↑ 34.0 34.1 Miles TS, Turker KS. Does reflex inhibition of motor units follow the "size principle"? Exp Brain Res 1986; 62: 443-445.
- ↑ Ellrich J, Hopf HC, Treede RD. Nociceptive masseter inhibitory reflexes evoked by laser radiant heat and electrical stimuli. Brain Res 1997; 764: 214-220.
- ↑ 36.0 36.1 Cruccu G, Romaniello A. Jaw-opening reflex after CO2 laser stimulation of the perioral region in man. Exp Brain Res 1998; 118: 564-568.
- ↑ Sessle BJ. Neural mechanisms and pathways in craniofacial pain. Can J Neurol Sci 1999; 26: 7-11.
- ↑ Wang K, Svensson P, Arendt-Nielsen L. Modulation of exteroceptive suppression periods in human jaw-closing muscles by local and remote experimental muscle pain. Pain 1999; 82: 253-262.
- ↑ Bendtsen L, Jensen R, Brennum J, Arendt-Nielsen L, Olesen J. Exteroceptive suppression periods in jaw-closing muscles. Variability and relation to experimental pain and sustained muscle contraction. Cephalalgia 1993; 13: 184-191.
- ↑ Bendtsen L, Jensen R, Brennum J, Arendt-Nielsen L, Olesen J. Exteroceptive suppression of temporal muscle activity is normal in chronic tension-type headache and not related to actual headache state. Cephalalgia 1996; 16: 251-256.
- ↑ Cadden SW, Newton JP. The effects of inhibitory controls triggered by heterotopic noxious stimuli on a jaw reflex evoked by perioral stimuli in man. Arch Oral Biol 1994; 39: 473-480.
- ↑ Schoenen J, Wang W, Gerard P. Modulation of temporalis muscle exteroceptive suppression by limb stimuli in normal man. Brain Res 1994; 657: 214-220.
- ↑ De Laat A, Svensson P, Macaluso GM. Are jaw and facial reflexes modulated during clinical or experimental orofacial pain? J Orofac Pain 1998; 12: 260-271.
- ↑ Schoenen J. Exteroceptive suppression of temporalis muscle activity: Methodological aspects and pathophysiological implications. Cephalalgia 1993; 13: 82-91.
- ↑ Darian-Smith I. Presynaptic component in the afferent inhibition observed within trigeminal brain-stem nuclei of the cat. J Neurophysiol 1965; 28: 695-709.
- ↑ Hu JW, Sessle BJ. Properties of functionally identified nociceptive and nonnociceptive facial primary afferents and presynaptic excitability changes induced in their brain stem endings by raphe and orofacial stimuli in cats. Exp Neurol 1988; 101: 385-399.
- ↑ Fitzgerald M. The contralateral input to the dorsal horn of the spinal cord in the decerebrate spinal rat. Brain Res 1982; 236: 257-287.