Role of Metabotropic Glutamate Receptors in Pain
Role of Metabotropic Glutamate Receptors in Pain
Glutamic Acid as a Key Excitatory Neurotransmitter
Glutamic acid represents the most widespread excitatory neurotransmitter in the Central Nervous System (CNS) and plays a key role in multiple functions. In recent years, the discovery of metabotropic glutamate receptors, a class of G-protein-coupled receptors, has led to a substantial body of experimental work aimed at clarifying the role of these receptors in both physiological activities and pathological processes within the CNS [1]. Glutamic acid receptors are divided into two main functional categories: ionotropic and metabotropic (mGlu) [2].
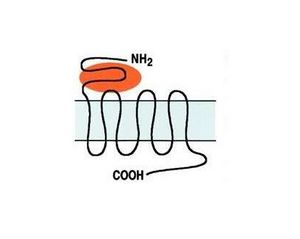
The metabotropic receptor group, which includes eight receptors, is further subdivided into three subgroups based on amino acid sequence homology, pharmacological profiles, and post-receptor signal transduction mechanisms (Fig. 1). Group I includes mGlu1 and mGlu5 receptors, activated by agonists specific to these receptors (DHPG and CHPG). Activation of Group I receptors stimulates the hydrolysis of membrane phosphoinositides through a G-protein-dependent mechanism. Group II (mGlu2, mGlu3) and Group III (mGlu4, mGlu6, mGlu7, mGlu8), activated by the selective agonists LY379268 and L-SOP respectively, share the mechanism of reducing cAMP synthesis [3].
mGlu receptors regulate neuronal excitability in various CNS regions, mainly by modulating the activation of ion channels. These receptors have been implicated in the pathogenesis of several CNS disorders, including epilepsy, ischemia, and neurodegenerative diseases [4].
Recent pharmacological, immunohistochemical, and in situ hybridization studies indicate that Group I mGlu receptors play a key role in nociceptive transmission. In addition to their role in pain transmission at the CNS level, both at the spinal and cortical-thalamic levels, glutamate has also been shown to excite peripheral nociceptive neurons, mediating responses partly related to ionotropic receptor activation and partly to mGlu receptor activation [5].
In recent years, pharmacological research has become more effective due to the synthesis of new, more selective molecules for individual receptors. Thus, metabotropic glutamate receptors represent a novel and promising target for analgesic therapy [6].
Role of Group I mGlu Receptors in Neurophysiopathological Mechanisms
In addition to playing an essential role in various physiological activities, glutamate has been implicated in the pathogenesis of several pathological conditions affecting the CNS. This is due primarily to its widespread distribution in all brain areas, but also to its ability to activate receptor subtypes linked to the activation of calcium-permeable ion channels, whose accumulation is known to be harmful to neurons. Indeed, excessive stimulation of glutamate receptors results in neuronal death, a phenomenon termed "excitotoxicity," which has been proposed as a pathogenic mechanism for a variety of CNS disorders, such as cerebral ischemia [7]. The concept of excitotoxicity has also been extended to epilepsy and chronic neurodegenerative disorders such as Parkinson's disease, Alzheimer's disease, and Huntington's chorea [8]. The exact role of glutamate in these pathological phenomena is not yet fully understood, whether it is a sufficient cause or a contributing factor to neuronal death. However, experimental evidence suggests that antagonists of glutamate receptors can exert neuroprotective actions.
The basal ganglia, and particularly the striatum, represent one of the most vulnerable brain regions to ischemic and excitotoxic insults, which are associated with excessive activation of glutamate receptors. The high energy demands required by neuronal cells are essential for maintaining proper ionic homeostasis. Within the striatum, GABAergic projection cells exhibit selective vulnerability to both energy deprivation and excitotoxic damage, whereas other cell types, such as cholinergic interneurons and nitric oxide synthase-positive interneurons, are particularly resistant [9]. However, the reasons for this differential vulnerability remain unclear.
In our laboratories, we have developed electrophysiological and fluorimetric techniques to evaluate neuronal responses to various exogenous stimuli. Specifically, male Wistar rats are sacrificed, and corticostriatal coronal slices (200-300 μm) are cut with a vibratome from blocks of brain tissue. A slice is transferred to a recording chamber, where it is immersed in a Krebs solution (2-3 ml/min), gassed with a 95% O2 - 5% CO2 mixture, and maintained at a constant temperature (32-33°C). For intracellular electrophysiological recordings, microelectrodes filled with KCl are used. Both current-clamp and voltage-clamp recordings are made using an Axoclamp-2A amplifier. For simultaneous fluorimetric and electrical recordings, the microelectrode is filled with a 1 mM bis-fura2 or 5 mM SBFI solution in 100 mM KCl for calcium or sodium measurements, respectively. The recording chamber is mounted on a microscope (Zeiss) equipped with a 60X objective (Olympus). Epillumination is provided by a 75W Xenon lamp, alternately filtered at 340 and 380 nm. Emission light is filtered at 500 nm, detected by a CCD camera, and images are analyzed using Ionvision software (ImproVision, UK).
It has been demonstrated that spiny neurons and cholinergic interneurons exhibit different sensitivities to glutamatergic agonists, both ionotropic and metabotropic [10]. These cells respond in opposite ways to energy deprivation [11], demonstrating a functional substrate, namely glutamatergic receptor sensitivity between spiny neurons and interneurons, capable of determining opposing behaviors between cell types. Furthermore, the functional interaction between ionotropic and mGlu receptors appears significantly different and is certainly one of the possible co-factors responsible for the different striatal neuronal vulnerabilities.
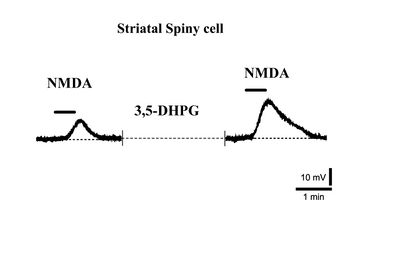
Indeed, it has been observed that while NMDA receptor responses in striatal spiny neurons are significantly potentiated by Group I mGlu agonists (Fig. 2) [12], this potentiation is never observed in cholinergic interneurons [13].
The development of selective pharmacological agents for ionotropic receptors has been recently hindered by the emergence of significant adverse effects in advanced stages of clinical trials. In contrast, there has been considerable progress in the development of selective agonists and antagonists for mGlu receptors. By developing drugs that can selectively inhibit glutamatergic transmission mediated by mGlu receptors, it may be possible to avoid the onset of side effects associated with ionotropic receptor activation. In particular, the most promising effort in the last 5-10 years has been the creation of receptor antagonists for Group I mGlu receptors, specifically mGlu1 and mGlu5.
Role of Group I mGlu Receptors in Central Pain Transmission
Experimental evidence suggests a significant involvement of excitatory amino acids, glutamate, and aspartate, in mediating both acute and chronic nociceptive transmission [14]. In fact, a large number of peripheral sensory fibers contain glutamate, including C fibers, and about 80% of Substance P fibers [15]. In the spinal cord, the response to brief acute mechanical or thermal stimuli primarily involves AMPA-type ionotropic receptors. If the stimulus is prolonged or if the frequency or intensity of the stimulus is increased, NMDA-type ionotropic receptors are also activated. This phenomenon results in an enhancement of the sensory response, a mechanism referred to as "sensitization." Sensitization is an increased response to a stimulus and has been experimentally reproduced in spinal cord preparations using the experimental paradigm known as "wind-up" [16].
The "wind-up" phenomenon is an increase in the number of action potentials generated by a neuron after successive stimuli during a train of impulses. It has been proposed that "wind-up" represents a central mechanism of hyperalgesia. It is interesting to note that both glutamate and the exogenous ionotropic NMDA agonist reproduce the phenomenon, while NMDA receptor antagonists are able to prevent it. Recent studies suggest the involvement of mGlu receptors in nociceptive transmission in line with their anatomical distribution, which shows the presence of Group I receptors (mGlu1 and mGlu5) in laminae I and II of the dorsal columns [17]. Similar to what has been observed with NMDA receptor antagonists, Group I mGlu antagonists have been shown to be effective in inhibiting wind-up [18].
This is confirmed by experimental evidence that mGlu agonists induce the phenomenon themselves. One of the experimental paradigms used to study the response to acute nociceptive stimuli in vitro involves a single robust electrical stimulation of the dorsal roots sufficient to recruit both A and C fibers. It has been shown that Group I mGlu antagonists, but not NMDA antagonists, are able to block the late component of potentials recorded from the ventral roots [19]. The late phase, often attributed to the activation of C fibers, is defined as "peptidergic" because it is blocked by neurokinin receptor antagonists. The early component of the potential is mainly mediated by AMPA-type ionotropic receptors and is not blocked by mGlu antagonists.
The activation of dorsal horn neurons is also produced by the application of mustard oil, a chemical irritant capable of activating C fibers. Mustard oil induces central sensitization of these neurons to afferent nociceptive stimuli, a process in many ways analogous to wind-up. This sensitization is prevented by pretreatment with Group I antagonists, confirming the hypothesis of Group I mGlu involvement in inflammatory pain. Together, these findings suggest that in addition to the role of NMDA receptors, Group I mGlu receptors are essential in generating the nociceptive response at the spinal cord level.
The thalamus represents a critical relay station for nociceptive information. One of the experimental models used to analyze pharmacological effects at the thalamic level involves the stimulation of the animal's vibrissae. It has been observed that the responses of thalamic neurons to painful thermal stimuli are reduced by Group I mGlu antagonists. It is important to note that the effect is selective, meaning that if the stimulus does not induce a painful sensation, it is not blocked [20]. A more recent study confirmed these findings using a selective mGlu1 antagonist, demonstrating for the first time a direct involvement of a specific mGlu receptor subtype in nociceptive response at the thalamic level [21]. This result is consistent with the receptor distribution of mGlu1 in the thalamus.
Similar to the thalamus, at the level of the somatosensory cortex, the sensory stimulation of the vibrissae is not altered by Group I mGlu antagonists, suggesting that these receptors are not involved in the transmission of simple mechanical stimuli. On the contrary, painful stimulation is blocked by Group I mGlu antagonists. Behavioral studies using the "hot-plate" model to study nociceptive reflexes have shown that intraventricular injection of Group I antagonists significantly reduces nociceptive responses [22].
The role of the periaqueductal gray (PAG) in nociceptive transmission is not fully understood. However, experimental evidence indicates that activation of Group I mGlu receptors in the PAG potentiates the antinociceptive activity of the descending pathway originating from this brain area. Indeed, recent studies indicate that intra-PAG administration of DHPG, a Group I mGlu agonist, reduces hyperalgesia induced by formalin injections [23]. This effect is thought to be linked to glutamate's ability, through mGlu activation, to prevent the establishment of the wind-up phenomenon.
Role of mGlu Receptors in Peripheral Nociceptive Transmission Mechanisms
The role of glutamate in the Peripheral Nervous System is still unclear. It has been shown that subcutaneous injection of glutamate in rats reduces the activation threshold for mechanical and thermal stimuli [24]. The application of antagonists for ionotropic glutamate receptors attenuates nociceptive levels in the formalin test, a model used to study inflammatory pain. Additionally, glutamate concentration increases in the cutaneous tissue after sciatic nerve stimulation and during the formalin test in rats [25]. Altogether, this data suggests that glutamate acts as an effective mediator of peripheral inflammation following tissue injury and that peripheral glutamate activates ionotropic receptors.
The study of mGlu receptor roles in pain transmission was, until a few years ago, confined to the dorsal columns of the spinal cord. Neurophysiological studies demonstrated that Group I receptors are involved in the hyperexcitability of these neurons following inflammatory stimuli. Behavioral studies indicate that intrathecal injection of Group I agonists induces hyperalgesia; additionally, intrathecal administration of Group I mGlu antagonists reduces inflammation and neuropathic pain [26][27]. Consistent with these studies, immunocytochemical research has localized Group I mGlu receptors in the dorsal horns of the spinal cord. These studies confirm the hypothesis that mGlu receptors are important in spinal sensitization following tissue injury.
More recently, the presence of mRNA for the Group I mGlu5 receptor has been demonstrated in the dorsal root ganglia of adult and neonatal rats. This finding suggests the possibility that, in addition to a role as a mediator of peripheral inflammation, glutamate may mediate primary afferent activity from the periphery through the activation of mGlu5 receptors [28]. In this study, the authors investigated the role of peripheral mGlu receptors in inflammatory pain and thermal nociception, demonstrating that both mGlu1 and mGlu5 receptors are expressed in unmyelinated nociceptive afferent fibers. Additionally, peripheral injection of agonists for these receptors was able to induce hyperalgesia through the activation of both mGlu1 and mGlu5. Finally, antagonists of these receptors blocked glutamate-induced hyperalgesia and significantly reduced inflammatory pain in the formalin test [29].
These findings suggest that Group I mGlu receptors mediate, at least in part, nociceptive transmission and thus may represent an interesting therapeutic target.
Conclusions
Understanding the functions of mGlu receptors in the Central and Peripheral Nervous Systems has made significant progress in recent years. In particular, the role of Group I mGlu receptors (mGlu1 and mGlu5) has been extensively investigated at the molecular, cellular, and behavioral levels, highlighting their importance in numerous physiological functions and pathological processes. Both mGlu1 and mGlu5 have been implicated in a variety of brain disorders, including epilepsy, pain, ischemia, and neurodegenerative diseases. The potential of these receptors to modulate glutamatergic transmission and their presence in multiple brain areas could, therefore, represent a genuine target for the treatment of neurological disorders.
- ↑ Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205-37.
- ↑ Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205-37.
- ↑ Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205-37.
- ↑ Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205-37.
- ↑ Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205-37.
- ↑ Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205-37.
- ↑ Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986 Feb;19(2):105-11.
- ↑ Albin RL, Greenamyre TJ. Alternative excitotoxic hypotheses. Neurology. 1992 Apr;42(4):733-8.
- ↑ Ferrante RJ, Kowall NW, Beal MF, Richardson EP Jr, Bird ED, Martin JB. Selective sparing of a class of striatal neurons in Huntington's disease. Science. 1985 Nov 1;230(4725):561-3.
- ↑ Calabresi P, Centonze D, Pisani A, Sancesario G, Gubellini P, Marfia GA, Bernardi G. Striatal spiny neurons and cholinergic interneurons express differential ionotropic glutamatergic responses and vulnerability: implications for ischemia and Huntington's disease. Ann Neurol. 1998 May;43(5):586-97.
- ↑ Calabresi P, Saiardi A, Pisani A, Baik JH, Centonze D, Mercuri NB, Bernardi G, Borrelli E. Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J Neurosci. 1997 Jun 15;17(12):4536-44.
- ↑ Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106(3):579-87.
- ↑ Calabresi P, Centonze D, Pisani A, Bernardi G. Metabotropic glutamate receptors and cell-type-specific vulnerability in the striatum: implication for ischemia and Huntington's disease. Exp Neurol. 1999 Jul;158(1):97-108.
- ↑ Dickenson AH, Chapman V, Green GM. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen Pharmacol. 1997 May;28(5):633-8.
- ↑ Battaglia G, Rustioni A. Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. J Comp Neurol. 1988 Nov 8;277(2):302-12.
- ↑ Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983 Dec 15-21;306(5944):686-8.
- ↑ Valerio A, Rizzonelli P, Paterlini M, Moretto G, Knopfel T, Kuhn R, Memo M, Spano P. mGluR5 metabotropic glutamate receptor distribution in rat and human spinal cord: a developmental study. Neurosci Res. 1997 May;28(1):49-57.
- ↑ Boxall SJ, Berthele A, Laurie DJ, Sommer B, Zieglgansberger W, Urban L, Tolle TR. Metabotropic glutamate receptor activation contributes to nociceptive reflex activity in the rat spinal cord in vitro. Neuroscience. 1996 Sep;74(1):13-20.
- ↑ Boxall SJ, Berthele A, Laurie DJ, Sommer B, Zieglgansberger W, Urban L, Tolle TR. Metabotropic glutamate receptor activation contributes to nociceptive reflex activity in the rat spinal cord in vitro. Neuroscience. 1996 Sep;74(1):13-20.
- ↑ Eaton SA, Jane DE, Jones PL, Porter RH, Pook PC, Sunter DC, Udvarhelyi PM, Roberts PJ, Salt TE, Watkins JC. Competitive antagonism at metabotropic glutamate receptors by (S)-4-carboxyphenylglycine and (RS)-alpha-methyl-4-carboxyphenylglycine. Eur J Pharmacol. 1993 Jan 15;244(2):195-7.
- ↑ Salt T, Turner JP. Reduction of sensory and metabotropic glutamate receptor responses in the thalamus by the novel metabotropic glutamate receptor-1-selective antagonist S-2-methyl-4-carboxy-phenylglycine. Neuroscience. 1998 Aug;85(3):655-8.
- ↑ Moroni F, Lombardi G, Thomsen C, Leonardi P, Attucci S, Peruginelli F, Torregrossa SA, Pellegrini-Giampietro DE, Luneia R, Pellicciari R. Pharmacological characterization of 1-aminoindan-1,5-dicarboxylic acid, a potent mGluR1 antagonist. J Pharmacol Exp Ther. 1997 May;281(2):721-9.
- ↑ Maione S, Oliva P, Marabese I, Palazzo E, Rossi F, Berrino L, Filippelli A. Periaqueductal gray matter metabotropic glutamate receptors modulate formalin-induced nociception. Pain. 2000 Mar;85(1-2):183-9.
- ↑ Jackson DL, Graff CB, Richardson JD, Hargreaves KM. Glutamate participates in the peripheral modulation of thermal hyperalgesia in rats. Eur J Pharmacol. 1995 Sep 25;284(3):321-5.
- ↑ de Groet M, van der Kooy D. Sensory neuron specific receptor activation reduces pain in rats. Nature. 2000.
- ↑ Fisher K, Coderre TJ. Hyperalgesia and allodynia induced by intrathecal (RS)-dihydroxyphenylglycine in rats. Neuroreport. 1998 Apr 20;9(6):1169-72.
- ↑ Fisher K, Fundytus ME, Cahill CM, Coderre TJ. Intrathecal administration of the mGluR compound, (S)-4CPG, attenuates hyperalgesia and allodynia associated with sciatic nerve constriction injury in rats.
- ↑ Bhave G, Karim F, Carlton SM, Gereau RW. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001 Apr;4(4):417-23.
- ↑ Bhave G, Karim F, Carlton SM, Gereau RW. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001 Apr;4(4):417-23.